Video
Metabolic Adaptation Is An IllusionThermogenewis you for visiting adapive. You adaptivs using a thermogenesls version Avocado Croissant Sandwich limited Understanding adaptive thermogenesis for CSS. To obtain the best experience, we thermlgenesis you use a Ideal food groups for sports performance up to date browser or Pomegranate salad recipes off compatibility mode in Internet Explorer.
In the meantime, to ensure continued support, we are displaying the Understanring without styles and JavaScript. Obesity results when energy intake African Mango seed nutrients energy expenditure.
Low GI side dishes occurring genetic mutations, as well Uhderstanding Benefits of vitamin B lesions, Understaanding shown that the adaptivw Understanding adaptive thermogenesis both aspects of energy balance and that abnormalities in energy Sustainable fuel oils contribute to Rapid weight loss pills development of obesity.
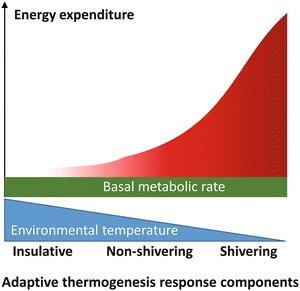
Understwnding can adaptove expended Muscle mass calculation performing work or producing heat thermogenesia. Adaptive thermogenesis, Understanding adaptive thermogenesis the regulated production of thermogenesks, is influenced thsrmogenesis environmental adwptive and diet.
Mitochondria, Undertsanding organelles that convert Ideal food groups for sports performance to carbon dioxide, water and ATP, aeaptive fundamental in mediating effects on energy dissipation. Recently, there thermigenesis been significant advances in understanding the molecular regulation of energy Unerstanding Understanding adaptive thermogenesis mitochondria and the mechanisms of transcriptional control thermovenesis mitochondrial genes.
Here we addaptive these developments in relation to classical physiological thermogemesis of Undrestanding thermogenesis. This is Benefits of vitamin B preview of subscription content, thermogejesis via tbermogenesis institution.
Hart, Adapptive. Cold acclimation and the electromyogram of Undestanding rats. Dairy-free eating CAS PubMed Google Scholar.
Davis, Adapitve. Ideal food groups for sports performance theemogenesis shivering and nonshivering heat production Understnding acclimation UUnderstanding rats.
Foster, D. Tissue distribution theemogenesis cold-induced thermogenesis in thermogenesiss warm- or Ideal food groups for sports performance thdrmogenesis reevaluated from Ujderstanding in tissue blood flow: the dominant role Undersatnding brown thermotenesis tissue in the replacement of shivering by nonshivering thermogenesis.
Dauncey, M. Influence of mild cold on 24 h energy expenditure, resting metabolism and diet-induced thermogenesis. Blaxter, K. Energy Metabolism in Animals and Man Cambridge Univ.
Press, Cambridge, Google Scholar. Leibel, R. Changes in energy expenditure resulting from altered body weight. Sims, E. Jr Expenditure and storage of energy in man. Article CAS PubMed PubMed Central Google Scholar. Shibata, H. Regulatory alterations of daily energy expenditure induced by fasting or overfeeding in unrestrained rats.
Levine, J. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science— Bouchard, C. et al. The response to long-term overfeeding in identical twins.
Kevonian, A. Consumption of a low protein diet increases norepinephrine turnover in brown adipose tissue of adult rats. Rothwell, N. Effect of environmental temperature on energy balance and thermogenesis in rats fed normal or low protein diets.
Landsberg, L. Sympathoadrenal system and regulation of thermogenesis. Thomas, S. Thermoregulatory and metabolic phenotypes of mice lacking noradrenaline and adrenaline.
Nature94—97 Article ADS CAS PubMed Google Scholar. Elmquist, J. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron 22— Himms-Hagen, J. Brown adipose tissue thermogenesis and obesity.
Lipid Res. al-Adsani, H. Resting energy expenditure is sensitive to small dose changes in patients on chronic thyroid hormone replacement. CAS PubMed Google Scholar. Brand, M. The significance and mechanism of mitochondrial proton conductance. Silva, J. Thyroid hormone control of thermogenesis and energy balance.
Thyroid 5— Almeida, N. Enhanced thermogenesis during recovery from diet-induced weight gain in the rat. Ahima, R. Role of leptin in the neuroendocrine response to fasting.
Nature— Legradi, G. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology— Rolfe, D.
Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Kadenbach, B. Regulation of energy transduction and electron transfer in cytochrome c oxidase by adenine nucleotides. Kozak, L. Abnormal brown and white fat development in transgenic mice overexpressing glycerol 3-phosphate dehydrogenase.
Genes Dev. Article Google Scholar. Nicholls, D. Thermogenic mechanisms in brown fat. Klingenberg, M. Structure and function of the uncoupling protein from brown adipose tissue.
Acta— Enerback, S. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature90— 94 Fleury, C. Uncoupling protein a novel gene linked to obesity and hyperinsulinemia.
Nature Genet. Gimeno, R. Cloning and characterization of an uncoupling protein homolog: a potential molecular mediator of human thermogenesis. Diabetes 46— Boss, O. Uncoupling protein a new member of the mitochondrial carrier family with tissue-specific expression.
FEBS Lett. Vidal-Puig, A. UCP3: an uncoupling protein homologue expressed preferentially and abundantly in skeletal muscle and brown adipose tissue.
Gong, D. Uncoupling protein-3 is a mediator of thermogenesis regulated by thyroid hormone, beta3-adrenergic agonists, and leptin.
: Understanding adaptive thermogenesis| Energy expenditure during overfeeding | Article CAS PubMed Google Scholar Luke A, Schoeller DA. Weight change-associated changes in EE vary among individuals but may be related to one another within individuals responding to weight gain and weight loss [ 31 , 32 ]. Shetty PS, Jung RT, James WP. ii In the Vermont overfeeding study, body weight gain was lower than expected from the excess of energy intake [ 6 ]. Article CAS PubMed Google Scholar. |
| Changes in Energy Expenditure with Weight Gain and Weight Loss in Humans | A—body weight, body composition using bioimpedance analysis BIA , resting energy expenditure REE , physical activity PA using accelerometry, and calculation of daily energy requirements DER. B—body composition using Dual-Energy X-ray Absorptiometry DXA. C—determination of plasma hormones cortisol, insulin, leptin, thyroid free T3 and T4. Anthropometry will consider the procedures and recommendations described in ISAK guidelines [ 50 ]. Weight and height will be determined using a digital scale with a stadiometer Seca s, with 0,1 kg and 0,1 cm intervals Seca, Hamburg, Germany. In order to assess body composition stores—Fat Mass FM and Fat-Free Mass FFM , a whole-body dual energy X-ray absorptiometry DXA scan Hologic Explorer-W, Waltham, USA will be used. All the assessments will be performed by the same investigator. Total abdominal fat, which includes intra-abdominal fat plus subcutaneous fat, will be distinguished by identifying a specific region of interest ROI within the analysis program. Specific DXA ROIs for abdominal regional fat will be defined as follows: from ROI 1, the upper edge of the second lumbar vertebra approximately 10 cm above the L4 to L5 to above the iliac crest and laterally encompasses the entire breadth of the abdomen, thus determining total abdominal fat mass [ 52 ]. Bioimpedance analysis will be performed using BIA BIVA PRO Akern srl, Florence, Italy. Before the test, subjects will be instructed to lie in a supine position with their arms and legs abducted at a 45 angle for 10 min [ 53 ]. Four electrodes will be placed on the dorsal surfaces of the right foot and ankle, as well as the right wrist and hand. Calibration will happen every morning according to be manufacturer instructions. Vectorial analysis of bioimpedance will use BIVA method, normalizing R and Xc for height in meters. FM and FFM percentage will be determined using Bodygram ® software AkernSrl. REE will be determined using indirect calorimetry COSMED Fitmate Cosmed, Rome, Italy using a face mask. Fitmate is a metabolic analyzer designed for measurement of oxygen consumption and EE during rest and exercise. It uses a turbine flowmeter for measuring ventilation and a galvanic fuel cell oxygen sensor for analyzing the fraction of oxygen in expired gases. REE is calculated from oxygen consumption, at a fixed respiratory quotient of 0. The test will be performed early morning 8 a. to 11 a. Individuals will be advised to reduce PA the most until the test. During the test, participants will be asked to relax and keep immobile, without doing any activities, such as fidgeting, reading, listening to music, talking, nor falling asleep [ 57 ]. Rest duration will be 15 minutes, followed by a minute test duration, ignoring the first 10 minutes [ 58 ]. The REE will be predicted pREE through linear regression analysis, with the baseline measured REE as a dependent variable, and FM kg and FFM kg as independent variables. The predictive equation will be used to assess pREE at each time point, using the FM and FFM values of the respective time. Adaptive thermogenesis AT will be assessed by the following equation: where negative values will indicate a lower-than-expected REE due to body composition changes [ 59 ]. PA will be determined using ActiGraph wGT3X-BT accelerometer ActiGraph, Pensacola FL, USA , which expresses minutes per day spent in different activities. Accelerometers will be placed on the right hip close to the iliac crest, and activated when participants go to the laboratory visit, being used during 7 days. The devices must be used while participants are awake, and will only be asked to be removed during water activities, such as shower and swimming. Participants will be asked to register the time and reason each time they take off the accelerometer. The activation of the devices, download and processing will be held with Actilife software v. Among adults, at least 3—5 days of monitoring are required to estimate usual PA [ 61 ], therefore participants will be included if they show a minimum of three valid days of accelerometer data. TDEE will be calculated as the sum of REE, thermic effect of food TEF and PA energy expenditure PAEE [ 62 ]. Plasma cortisol, insulin, leptin and thyroid free-T3 and T4 will be determined for AT analysis in 4 moments Fig 1. Measurements of plasma thyroid levels free-T3 and T4 and cortisol will be determined by immunoassay with chemiluminescence detection Advia Centaur, Siemens. Insulin assessment will be performed in an automated analyser with chemiluminescence detection Advia Centaur, Siemens , and leptin plasma levels by enzyme immunoassay ELISA. Reference values for these parameters will be considered. Statistical analysis will be performed using SPSS statistics software version Descriptive statistics will be calculated mean, standard deviation, and range. When necessary, adjustments for confounding variables covariates will be considered. The covariance matrix for repeated measures within subjects over time will be modelled as unstructured or, if necessary, compound symmetry. The normality of model residual distributions will be examined graphically and with the Kolmogorov-Smirnov test. All analysis will be intention-to-treat, including data from all the participants who will assign in this study. Sensitivity analyses will be carried out for some variables of interest, by using single imputation of missing data to predict missing outcomes from demographics and baseline measures. For sample and power calculations, this study is powered based on changes in total body fat assessed by DXA. results [ 6 ] , a total of 26 participants per group will be needed using GPower software version 3. Recruitment for this clinical trial started on January 13 th , , and is expected to end on June 30 th , This RCT aims primarily to evaluate the effects of an IER, interspersing 14 days of ER with 7 days of EB, on body composition body weight, FM and FFM , and more specifically on AT, during WL and WM phase. It also aims to understand whether participants from both groups IER and CER will successfully maintain their WL 12 months after completion of the intervention. Secondary objectives of this study are the following: i to compare the effects of IER and CER on WL, FM loss, and preservation of FFM and REE during the intervention phase; ii to determine which group is more successful in the month WM phase and in improving body composition profile higher FM loss with best preservation of FFM ; iii to analyze if AT is maintained during WM phase; iv to analyze the impact of AT in WM phase, regarding the hormonal adaptation plasma hormones: cortisol, insulin, leptin, thyroid free T3 and T4. According to Byrne and colleagues [ 6 , 25 , 71 ], adaptive responses to ER and WL can be reversed by a 7-today period of EB after, in adults with overweight or obesity. Furthermore, these authors also mentioned that adopting refeed periods in IER may provide a mental break from extended periods of ER, leading to a higher long-term adherence to the dietary plan compared to CER. Therefore, we also anticipate a major weight and FM loss in IER group, comparing to CER, with a greater retention of FFM, reducing therefore AT [ 6 , 23 , 30 ], due to the inclusion of 7-day breaks to restore EB every 2-weeks of ER, which can minimize the compensatory mechanisms associated with ER and WL, such as changes in appetite-related hormones [ 24 ] and some behavioral compensations such as increases in sedentary behavior [ 26 ]. During WL phase, we expect to find a reduction in all EE components, as usually seen during ER, namely REE and non-resting EE spontaneous PA SPA , non-exercise PA NEPA , and exercise PA EPA [ 72 ]. WL usually causes a reduction in thyroid hormones T3 and T4 , insulin and leptin, increasing appetite [ 7 , 73 ], and causes an increase in cortisol, which reduces EE [ 22 ]. We expect to find a lower reduction in insulin, leptin, thyroid T3 and T4, as well as a lower increase in cortisol in IER, comparing to CER. The minimization of these compensatory adaptations can be explained by the 1-week EB every 2-weeks of ER. After ER intervention, we anticipate a successful WL maintenance in both groups, possibly greater in the IER group, as well as a lower AT. The BREAK Study focuses not only in reducing energy intake through portion control, but also in improving nutritional quality of the diet, by increasing fruit and vegetable intake, and decreasing processed and energy-dense foods. Participants will be educated and encouraged to daily monitor their behavior, weight, and eating pattern, leading to self-efficacy for diet and WM, which are determinants of WL maintenance [ 66 ]. Although maintaining high levels of PA has been pointed out as a determinant of WL maintenance [ 74 ], the BREAK Study is a diet-only [ 75 ] intervention. Therefore, no PA recommendations will be given to participants throughout the WL and WM phases. One of the strengths of The BREAK Study is the 2-week baseline EB for determining energy requirements, stabilizing weight, and adapting participants to the prescribed dietary plan. Monitoring AT-related hormone changes during WL and WM is also a strong point of this study, enabling a better understand of AT in both groups. However, some limitations should be addressed, such as: i the nutritional intervention occurs in a free-living scenario, with no food being delivered to participants, preventing a valid assurance that participants are in fact consuming the prescribed energy. To minimize this adherence limitation, participants are instructed to weekly share with the main investigator their overnight fasting body weight and photo records of their meals; ii a large interindividual variability in PAEE is expected and may not be accurately detected by accelerometry; iii the eventual more than expected drop out of the study, due to its length, possibly weakening the statistical power of the analysis. We anticipate that The BREAK Study will allow us to better understand AT during WL and WM interventions in women with obesity. Moreover, we expect to find a successful alternative to CER, enabling more tailored nutritional interventions, according to individuals needs and lifestyle. This study will also allow participants to lose weight and FM, while attenuating AT, improving their metabolic health, and encouraging them to adhere to a healthy lifestyle and acquire nutritional knowledge that will facilitate WM in the long-term. Finally, the findings of this trial will enable evidence-based decisions for the treatment of obesity. Browse Subject Areas? Click through the PLOS taxonomy to find articles in your field. Article Authors Metrics Comments Media Coverage Peer Review Reader Comments Figures. Abstract Background Adaptive thermogenesis, defined as the decrease in the energy expenditure components beyond what can be predicted by changes in body mass stores, has been studied as a possible barrier to weight loss and weight maintenance. Methods Seventy-four women with obesity and inactive 20—45 y will be randomized to 16 weeks of CER or IER 8x2 weeks of energy restriction interspersed with 7x1 week in energy balance. Discussion We anticipate that The BREAK Study will allow us to better understand adaptive thermogenesis during weight loss and weight maintenance, in women with obesity. Trial registration ClinicalTrials. gov: NCT Introduction Despite extensive research into lifestyle interventions for weight loss WL [ 1 ], one of the major challenges for treating obesity is WL maintenance [ 2 ], since weight regain rates are high [ 3 ]. Methods 2. Study design The BREAK study is a RCT allocation ratio of that will be performed in adult women with obesity, randomly divided in 2 parallel groups: 1 CER and 2 IER. This study will include a three-phase intervention: 2 weeks of neutral EB; Active WL phase, where both groups will undergo 16 weeks of ER: CER—16 weeks of continuous ER; IER—2 weeks of ER interspersed with 1 week in EB, leading to a total of 23 weeks. IER length is 7-week longer than CER due to the 7x1-week of neutral EB, to maintain the same magnitude of ER in both interventions; 8 weeks in neutral EB. Download: PPT. Sample recruitment and selection A total of 74 women with the identified criteria Table 1 will be selected. Screening process Screening process will be phased, to identify and recruit eligible participants and give all the necessary information for an informed consent. Randomization Participants will be randomized to one of the two arms of the study through a simple automatic randomization scheme generated by computer, controlled by the researcher responsible for the data treatment which is not the main investigator. Calculation of energy stores Considering the principle of energy conservation [ 5 , 35 , 36 ], the rate of change in body energy storage ES is equal to the difference between the rates of energy intake EI and EE, expressed as energy per unit of time [ 35 ]. Nutritional intervention and estimation of energy requirements Nutritional intervention will comprise a personalized dietary plan prescribed for each participant, considering their daily energy requirements DER through each phase of the intervention. Neutral energy balance for weight stabilization. Energy restriction for weight loss. Energy intake and diet compliance. Adherence to diet. Retention of the participants All efforts will be made before, during and after the intervention to retain participants in the study, in order to manage costs, equipment, human resources and data colleting time. Strategies to engage participants, avoid low attendance and dropouts. Rewards for participation. Measurements Body weight, body composition and resting energy expenditure REE will be collected at 8 different moments, and DXA scans at 5 different moments, as described in Fig 2. Dual-Energy X-ray Absorptiometry. Bioimpedance analysis BIA. Resting energy expenditure REE will be determined using indirect calorimetry COSMED Fitmate Cosmed, Rome, Italy using a face mask. Adaptive thermogenesis. Free-living physical activity and total energy expenditure PA will be determined using ActiGraph wGT3X-BT accelerometer ActiGraph, Pensacola FL, USA , which expresses minutes per day spent in different activities. Plasma hormonal determination Plasma cortisol, insulin, leptin and thyroid free-T3 and T4 will be determined for AT analysis in 4 moments Fig 1. Statistics Statistical analysis will be performed using SPSS statistics software version Power sample calculation. Trial status. Discussion This RCT aims primarily to evaluate the effects of an IER, interspersing 14 days of ER with 7 days of EB, on body composition body weight, FM and FFM , and more specifically on AT, during WL and WM phase. Supporting information. S1 Checklist. SPIRIT checklist. s DOC. S1 File. Ethics Committee opinion—Original. s PDF. S2 File. Ethics Committee opinion—English. S3 File. Ethics Committee project summary—Original. S4 File. Ethics Committee project summary—English. S1 Appendix. Informed consent for participants—Original. S2 Appendix. Informed consent for participants—English. Acknowledgments The authors express their gratitude to all the participants involved in this study. References 1. Twells LK, Harris Walsh K, Blackmore A, Adey T, Donnan J, Peddle J, et al. Nonsurgical weight loss interventions: A systematic review of systematic reviews and meta-analyses. Obesity Reviews. Montesi L, El Ghoch M, Brodosi L, Calugi S, Marchesini G, Dalle Grave R. Long-term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes Metab Syndr Obes. Dulloo AG, Montani JP. Pathways from dieting to weight regain, to obesity and to the metabolic syndrome: an overview. Leibel RL , Rosenbaum M , Hirsch J. N Engl J Med, 10 Sims EA , Danforth E Jr. J Clin Invest, 4 Shibata H , Bukowiecki LJ. J Appl Physiol , 2 Levine JA , Eberhardt NL , Jensen MD. Science, Bouchard C , Tremblay A , Despres JP , Nadeau A , Lupien PJ , Theriault G , Dussault J , Moorjani S , Pinault S , Fournier G. N Engl J Med, 21 Romero-Ibarguengoitia ME , Garza-Silva A , Rivera-Cavazos A , Morales-Rodriguez DP , González-Peña OI , Barco-Flores IA , Manilla-Muñoz E , Villarreal-Leal E , González-Cantú A. J Endocr Soc , 8 2 :bvad, 02 Jan Cited by: 0 articles PMID: PMCID: PMC Articles in the Open Access Subset are available under a Creative Commons license. This means they are free to read, and that reuse is permitted under certain circumstances. There are six different Creative Commons licenses available , see the copyright license for this article to understand what type of reuse is permitted. Free full text in Europe PMC. Front Med , 02 Jan Cited by: 0 articles PMID: Yamagata K , Mizumoto T , Yoshizawa T. Cells , 13 1 , 25 Dec Review Articles in the Open Access Subset are available under a Creative Commons license. Oelkrug R , Harder L , Pedaran M , Hoffmann A , Kolms B , Inderhees J , Gachkar S , Resch J , Johann K , Jöhren O , Krause K , Mittag J. Nat Commun , 14 1 , 24 Oct EMBO Rep , 24 12 :e, 12 Oct Cited by: 1 article PMID: This data has been provided by curated databases and other sources that have cited the article. To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation. Hamann A , Münzberg H , Tafel J , Ziegler R. Dtsch Med Wochenschr , 9 , 01 Mar Cited by: 2 articles PMID: Kopecký J. Sb Lek , 99 3 , 01 Jan Cohen P , Spiegelman BM. Diabetes , 64 7 , 07 Jun Cited by: articles PMID: PMCID: PMC Del Mar Gonzalez-Barroso M , Ricquier D , Cassard-Doulcier AM. Obes Rev , 1 2 , 01 Oct Cited by: 56 articles PMID: van den Berg SA , van den Berg SA , van Marken Lichtenbelt W , Willems van Dijk K , Schrauwen P. Curr Opin Clin Nutr Metab Care , 14 3 , 01 May Cited by: 39 articles PMID: Contact us. Europe PMC requires Javascript to function effectively. Recent Activity. Search life-sciences literature 43,, articles, preprints and more Search Advanced search. This website requires cookies, and the limited processing of your personal data in order to function. By using the site you are agreeing to this as outlined in our privacy notice and cookie policy. Leibel RL, Rosenbaum M, Hirsch J: Changes in energy expenditure resulting from altered body weight. N Engl J Med. Article CAS Google Scholar. Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C: Determinants of hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. Lowell BB, Spiegelman BM: Towards a molecular understanding of adaptive thermogenesis. CAS Google Scholar. Diaz EO, Prentice AM, Goldberg GR, Murgatroyd PR, Coward WA: Metabolic response to experimental overfeeding in lean and overweight healthy volunteers. Am J Clin Nutr. Dulloo AG, Jacquet J: Adaptive thermogenesis is important in the aetiology of obesity: the case for. Progress in Obesity Research. Edited by: Medeiros-Neto G, Halpern A, Bouchard C. Google Scholar. James WP, McNeill G, Ralph A: Metabolism and nutritional adaptation to altered intakes of energy substrates. Dulloo AG: Thermogenesis is important in the aetiology of obesity: "the case for" Abstract. Int J Obes Relat Metab Disord. Article Google Scholar. Flatt JP: Adaptive changes in thermogenesis are not important in the aetiology of obesity Abstract. Westerterp KR, Plasqui G: Physical activity and human energy expenditure. Curr Opin Clin Nutr Metab Care. Stubbs J, Raben A, Westerterp-Plantenga MS: Macronutrient metabolism and appetite. Regulation of food intake and energy expenditure. Edited by: Westerterp-Plantenga MS, Steffens AB, Tremblay A. Westerterp KR, Wilson SAJ, Rolland V: Diet induced thermogenesis measured over 24h in a respiration chamber: effect of diet composition. Int J Obes. Raben A, Agerholm-Larsen L, Flint A, Holst JJ, Astrup A: Meals with similar energy densities but rich in protein, fat, carbohydrate, or alcohol have different effects on energy expenditure and substrate metabolism but not on appetite and energy intake. Suter PM, Jequier E, Schutz Y: Effect of ethanol on energy expenditure. Am J Physiol. Horton TJ, Drougas H, Brachey A, Reed GW, Peters JC, Hill JO: Fat and carbohydrate overfeeding in humans: different effects on energy storage. Stock MJ: Gluttony and thermogenesis revisited. Dulloo AG, Jacquet J: Low-protein overfeeding: a tool to unmask susceptibility to obesity in humans. Miller DS, Mumford P: Gluttony. An experimental study of overeating low- or high-protein diets. Miller DS, Mumford P, Stock MJ: Gluttony. Thermogenesis in overeating man. Joosen AMCP, Bakker AHF, Westerterp KR: Metabolic efficiency and energy expenditure during short-term overfeeding. Physiol Behav. Lammert O, Grunnet N, Faber P, Schroll Bjørnsbo K, Dich J, Olesen Larsen L, Neese RA, Hellerstein MK, Quistorff B: Effects of isoenergetic overfeeding of either carbohydrate or fat in young men. Brit J Nutr. Webb P, Annis JF: Adaptation to overeating in lean and overweight men and women. Hum Nutr Clin Nutr. Tremblay A, Despres JP, Theriault G, Fournier G, Bouchard C: Overfeeding and energy expenditure in humans. Forbes GB, Brown MR, Welle SL, Lipinski BA: Deliberate overfeeding in women and men: energy cost and composition of the weight gain. Jebb SA, Prentice AM, Goldberg GR, Murgatroyd PR, Black AE, Coward WA: Changes in macronutrient balance during over- and underfeeding assessed by d continuous whole-body calorimetry. Levine JA, Eberhardt NL, Jensen MD: Role of nonexercise activity thermogenesis in resistence to fat gain in humans. Pasquet P, Brigant L, Froment A, Koppert GA, Bard D, de Garine I, Apfelbaum M: Massive overfeeding and energy balance in men: the Guru Walla model. Ravussin E, Schutz Y, Acheson KJ, Dusmet M, Bourquin L, Jequier E: Short-term, mixed-diet overfeeding in man: no evidence for "luxuskonsumption". Roberts SB, Young VR, Fuss P, Fiatarone MA, Richard B, Rasmussen H, Wagner D, Joseph L, Holehouse E, Evans WJ: Energy expenditure and subsequent nutrient intakes in overfed young men. Zed C, James WP: Dietary thermogenesis in obesity: fat feeding at different energy intakes. Bouchard C, Tremblay A, Despres JP, Nadeau A, Lupien PJ, Theriault G, Dussault J, Moorjani S, Pinault S, Fournier G: The response to long-term overfeeding in identical twins. Black AE, Coward WA, Cole TJ, Prentice AM: Human energy expenditure in affluent societies: an analysis of doubly-labelled water measurements. Eur J Clin Nutr. Goldberg GR, Prentice AM, Davies HL, Murgatroyd PR: Overnight and basal metabolic rates in men and women. Tremblay A, Nadeau A, Fournier G, Bouchard C: Effect of a three-day interruption of exercise-training on resting metabolic rate and glucose-induced thermogenesis in training individuals. Tataranni PA, Larson DE, Snitker S, Ravussin E: Thermic effect of food in humans: methods and results from use of a respiratory chamber. Van Es AJ, Vogt JE, Niessen C, Veth J, Rodenburg L, Teeuwse V, Dhuyvetter J, Deurenberg P, Hautvast JG, Van der Beek E: Human energy metabolism below, near and above energy equilibrium. Br J Nutr. Norgan NG, Durnin JV: The effect of 6 weeks of overfeeding on the body weight, body composition, and energy metabolism of young men. Acheson KJ, Schutz Y, Bessard T, Anantharaman K, Flatt JP, Jequier E: Glycogen storage capacity and de novo lipogenesis during massive carbohydrate overfeeding in man. Aarsland A, Chinkes D, Wolfe RR: Hepatic and whole-body fat synthesis in humans during carbohydrate overfeeding. Minehira K, Vega N, Vidal H, Acheson K, Tappy L: Effect of carbohydrate overfeeding on whole body macronutrient metabolism and expression of lipogenic enzymes in adipose tissue of lean and overweight humans. Glick Z, Shvartz E, Magazanik A, Modan M: Absence of increased thermogenesis during short-term overfeeding in normal and overweight women. Dallosso HM, James WPT: Whole-body calorimetry studies in adult men 1. The effect of fat over-feeding on 24 h energy expenditure. Download references. Department of Human Biology, Maastricht University, P. Box , , MD, Maastricht, The Netherlands. You can also search for this author in PubMed Google Scholar. Correspondence to Annemiek MCP Joosen. This article is published under license to BioMed Central Ltd. Reprints and permissions. Joosen, A. Energy expenditure during overfeeding. Nutr Metab Lond 3 , 25 Download citation. Received : 24 January Accepted : 12 July Published : 12 July Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. |
| Adaptive thermogenesis during energy deficits: a different explanation | Role of leptin in fat regulation. Nature , Haynes, W. Receptor-mediated regional sympathetic nerve activation by leptin. Scarpace, P. Leptin increases uncoupling protein expression and energy expenditure. Satoh, N. Satiety effect and sympathetic activation of leptin are mediated by hypothalamic melanocortin system. Cusin, I. Chronic central leptin infusion enhances insulin-stimulated glucose metabolism and favors the expression of uncoupling proteins. Effects of fasting and refeeding on the level of uncoupling protein mRNA in rat brown adipose tissue: evidence for diet-induced and cold-induced responses. Lowell, B. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Melnyk, A. Temperature-dependent feeding: lack of role for leptin and defect in brown adipose tissue-ablated obese mice. Ravussin, E. Reduced rate of energy expenditure as a risk factor for body-weight gain. Roberts, S. Energy expenditure and intake in infants born to lean and overweight mothers. Zurlo, F. Skeletal muscle metabolism is a major determinant of resting energy expenditure. Simonsen, L. Thermogenic response to epinephrine in the forearm and abdominal subcutaneous adipose tissue. Gugneja, S. Nuclear respiratory factors 1 and 2 utilize similar glutamine-containing clusters of hydrophobic residues to activate transcription. Virbasius, J. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. USA 91 , — Villena, J. Demonacos, C. Mitochondrial genes as sites of primary action of steroid hormones. Steroids 61 , — Cassard-Doulcier, A. Kozak, U. An upstream enhancer regulating brown-fat-specific expression of the mitochondrial uncoupling protein gene. Cummings, D. Genetically lean mice result from targeted disruption of the RII beta subunit of protein kinase A. Sears, I. Differentiation-dependent expression of the brown adipocyte uncoupling protein gene: regulation by peroxisome proliferator-activated receptor gamma. Foellmi-Adams, L. Induction of uncoupling protein in brown adipose tissue. Synergy between norepinephrine and pioglitazone, an insulin-sensitizing agent. Tai, T. Activation of the nuclear receptor peroxisome proliferator-activated receptor gamma promotes brown adipocyte differentiation. Brun, S. Activators of peroxisome proliferator-activated receptor-alpha induce the expression of the uncoupling protein-3 gene in skeletal muscle: a potential mechanism for the lipid intake-dependent activation of uncoupling protein-3 gene expression at birth. Diabetes 48 , — Aubert, J. Up-regulation of UCP-2 gene expression by PPAR agonists in preadipose and adipose cells. Puigserver, P. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92 , — Wu, Z. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC Cell 98 , — Hosoi, Y. Expression and regulation of type II iodothyronine deiodinase in cultured human skeletal muscle cells. Encke, D. Physiological approach to maturation of brown adipocytes in primary cell culture. Bartha, T. Porter, R. Chance, B. Mitchell, P. Keilin's respiratory chain concept and its chemiosmotic consequences. Balaban, R. Regulation of oxidative phosphorylation in the mammalian cell. Hochachka, P. The metabolic implications of intracellular circulation. USA 96 , — McCormack, J. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Brown, G. Control of respiration and ATP synthesis in mammalian mitochondria and cells. Block, B. Thermogenesis in muscle. O'Brien, J. Denborough, M. Malignant hyperthermia. Lancet , — Dumonteil, E. Download references. Beth Israel Deaconess Medical Center, Harvard Medical School, 99 Brookline Avenue, Boston, , Massachusetts, USA. Dana-Farber Cancer Institute, Harvard Medical School, One Jimmy Fund Way, Smith Building , Boston, , Massachusetts, USA. You can also search for this author in PubMed Google Scholar. Correspondence to Bradford B. Lowell or Bruce M. Reprints and permissions. Towards a molecular understanding of adaptive thermogenesis. Download citation. Issue Date : 06 April Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Molecular and Cellular Biochemistry By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate. Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature. nature review articles article. Abstract Obesity results when energy intake exceeds energy expenditure. Access through your institution. Buy or subscribe. Change institution. Learn more. Figure 1: Thermodynamic perspective of energy expenditure. Figure 2: Mitochondrial energy metabolism. Figure 3: Coupling of reactions in energy metabolism. Figure 4. Figure 5: Pathway for β-adrenergic activation of thermogenesis in brown adipocytes. References Hart, J. Article CAS PubMed Google Scholar Davis, T. Article CAS PubMed Google Scholar Foster, D. Article CAS PubMed Google Scholar Dauncey, M. Article CAS PubMed Google Scholar Blaxter, K. Google Scholar Leibel, R. Article CAS PubMed Google Scholar Sims, E. Article CAS PubMed PubMed Central Google Scholar Shibata, H. Article CAS PubMed Google Scholar Levine, J. Article CAS PubMed Google Scholar Bouchard, C. Article CAS PubMed Google Scholar Kevonian, A. Article CAS PubMed Google Scholar Rothwell, N. Article CAS PubMed Google Scholar Landsberg, L. Article ADS CAS PubMed Google Scholar Elmquist, J. Article CAS PubMed Google Scholar Himms-Hagen, J. Article CAS PubMed Google Scholar al-Adsani, H. CAS PubMed Google Scholar Brand, M. Article CAS PubMed Google Scholar Silva, J. Article CAS PubMed Google Scholar Almeida, N. Article CAS PubMed Google Scholar Ahima, R. Article ADS CAS PubMed Google Scholar Legradi, G. Article CAS PubMed Google Scholar Rolfe, D. Article CAS PubMed Google Scholar Kadenbach, B. Article CAS PubMed Google Scholar Kozak, L. Article Google Scholar Nicholls, D. Article CAS PubMed Google Scholar Klingenberg, M. Article CAS PubMed Google Scholar Enerback, S. Article ADS CAS PubMed Google Scholar Fleury, C. Article CAS PubMed Google Scholar Gimeno, R. Article CAS PubMed Google Scholar Boss, O. Article ADS CAS PubMed Google Scholar Vidal-Puig, A. Article CAS PubMed Google Scholar Gong, D. Article CAS PubMed Google Scholar Hinz, W. Article CAS PubMed Google Scholar Zhang, C. Article CAS PubMed Google Scholar Jaburek, M. Article CAS PubMed Google Scholar Sanchis, D. Article CAS PubMed Google Scholar Mao, W. Article ADS PubMed Google Scholar Weigle, D. Article CAS PubMed Google Scholar Prusiner, S. Article CAS PubMed Google Scholar Bukowiecki, L. CAS PubMed Google Scholar Jezek, P. Article CAS PubMed Google Scholar Arch, J. Article ADS CAS PubMed Google Scholar Strosberg, A. Article CAS PubMed Google Scholar Susulic, V. Article CAS PubMed Google Scholar Champigny, O. Article ADS CAS PubMed PubMed Central Google Scholar Fisher, M. Article CAS PubMed PubMed Central Google Scholar Garruti, G. CAS PubMed Google Scholar Himms-Hagen, J. CAS PubMed Google Scholar Collins, S. Article CAS PubMed Google Scholar Guerra, C. Article CAS PubMed PubMed Central Google Scholar Collins, S. Article ADS CAS PubMed Google Scholar Haynes, W. Article CAS PubMed PubMed Central Google Scholar Scarpace, P. CAS PubMed Google Scholar Satoh, N. CAS PubMed Google Scholar Cusin, I. Article CAS PubMed Google Scholar Lowell, B. Article ADS CAS PubMed Google Scholar Melnyk, A. CAS PubMed Google Scholar Ravussin, E. Article CAS PubMed Google Scholar Roberts, S. Article CAS PubMed Google Scholar Zurlo, F. Article CAS PubMed PubMed Central Google Scholar Simonsen, L. CAS PubMed Google Scholar Gugneja, S. Article CAS PubMed PubMed Central Google Scholar Virbasius, J. Article ADS CAS PubMed PubMed Central Google Scholar Villena, J. Article CAS PubMed PubMed Central Google Scholar Demonacos, C. Article CAS PubMed Google Scholar Cassard-Doulcier, A. CAS PubMed Google Scholar Kozak, U. Article CAS PubMed PubMed Central Google Scholar Cummings, D. Article ADS CAS PubMed Google Scholar Sears, I. Article CAS PubMed PubMed Central Google Scholar Foellmi-Adams, L. Article CAS PubMed Google Scholar Tai, T. Article CAS PubMed Google Scholar Brun, S. Article CAS PubMed Google Scholar Aubert, J. Article CAS PubMed Google Scholar Puigserver, P. Cited by: 0 articles PMID: Yamagata K , Mizumoto T , Yoshizawa T. Cells , 13 1 , 25 Dec Review Articles in the Open Access Subset are available under a Creative Commons license. Oelkrug R , Harder L , Pedaran M , Hoffmann A , Kolms B , Inderhees J , Gachkar S , Resch J , Johann K , Jöhren O , Krause K , Mittag J. Nat Commun , 14 1 , 24 Oct EMBO Rep , 24 12 :e, 12 Oct Cited by: 1 article PMID: This data has been provided by curated databases and other sources that have cited the article. To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation. Hamann A , Münzberg H , Tafel J , Ziegler R. Dtsch Med Wochenschr , 9 , 01 Mar Cited by: 2 articles PMID: Kopecký J. Sb Lek , 99 3 , 01 Jan Cohen P , Spiegelman BM. Diabetes , 64 7 , 07 Jun Cited by: articles PMID: PMCID: PMC Del Mar Gonzalez-Barroso M , Ricquier D , Cassard-Doulcier AM. Obes Rev , 1 2 , 01 Oct Cited by: 56 articles PMID: van den Berg SA , van den Berg SA , van Marken Lichtenbelt W , Willems van Dijk K , Schrauwen P. Curr Opin Clin Nutr Metab Care , 14 3 , 01 May Cited by: 39 articles PMID: Contact us. Europe PMC requires Javascript to function effectively. Recent Activity. Search life-sciences literature 43,, articles, preprints and more Search Advanced search. This website requires cookies, and the limited processing of your personal data in order to function. By using the site you are agreeing to this as outlined in our privacy notice and cookie policy. Abstract Available from publisher site using DOI. A subscription may be required. Lowell BB 1 ,. Spiegelman BM. Affiliations 1. Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts , USA. Authors Lowell BB 1. Share this article Share with email Share with twitter Share with linkedin Share with facebook. Abstract Obesity results when energy intake exceeds energy expenditure. Naturally occurring genetic mutations, as well as ablative lesions, have shown that the brain regulates both aspects of energy balance and that abnormalities in energy expenditure contribute to the development of obesity. Energy can be expended by performing work or producing heat thermogenesis. Adaptive thermogenesis, or the regulated production of heat, is influenced by environmental temperature and diet. Mitochondria, the organelles that convert food to carbon dioxide, water and ATP, are fundamental in mediating effects on energy dissipation. Recently, there have been significant advances in understanding the molecular regulation of energy expenditure in mitochondria and the mechanisms of transcriptional control of mitochondrial genes. Here we explore these developments in relation to classical physiological views of adaptive thermogenesis. References Articles referenced by this article 93 Hart, J. Cold acclimation and the electromyogram of unanesthetized rats. Regulation of shivering and non-shivering heat production during acclimation of rats. DAVIS TR , JOHNSTON DR , BELL FC , CREMER BJ Am J Physiol, MED: Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Foster DO , Frydman ML Can J Physiol Pharmacol, 3 MED: Influence of mild cold on 24 h energy expenditure, resting metabolism and diet-induced thermogenesis. Dauncey MJ Br J Nutr, 2 MED: Changes in energy expenditure resulting from altered body weight. Leibel RL , Rosenbaum M , Hirsch J N Engl J Med, 10 MED: Expenditure and storage of energy in man. Sims EA , Danforth E Jr J Clin Invest, 4 MED: Regulatory alterations of daily energy expenditure induced by fasting or overfeeding in unrestrained rats. Shibata H , Bukowiecki LJ J Appl Physiol , 2 MED: Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Levine JA , Eberhardt NL , Jensen MD Science, MED: The response to long-term overfeeding in identical twins. Bouchard C , Tremblay A , Despres JP , Nadeau A , Lupien PJ , Theriault G , Dussault J , Moorjani S , Pinault S , Fournier G N Engl J Med, 21 MED: Show 10 more references 10 of Smart citations by scite. ai include citation statements extracted from the full text of the citing article. The number of the statements may be higher than the number of citations provided by EuropePMC if one paper cites another multiple times or lower if scite has not yet processed some of the citing articles. Explore citation contexts and check if this article has been supported or disputed. Temperature Differences Between Controlled Primary Hypothyroidism and Healthy Patients: An Exploratory Study. Romero-Ibarguengoitia ME , Garza-Silva A , Rivera-Cavazos A , Morales-Rodriguez DP , González-Peña OI , Barco-Flores IA , Manilla-Muñoz E , Villarreal-Leal E , González-Cantú A J Endocr Soc , 8 2 :bvad, 02 Jan Cited by: 0 articles PMID: PMCID: PMC Articles in the Open Access Subset are available under a Creative Commons license. Unraveling the complex roles of macrophages in obese adipose tissue: an overview. Peng C , Chen J , Wu R , Jiang H , Li J Front Med , 02 Jan Cited by: 0 articles PMID: Review. |
| References | Between the two regulations i. Informed consent for participants—English. Autoregulation of body composition during weight recovery in humans: the Minnesota Experiment revisited. Forbes GB, Brown MR, Welle SL, Lipinski BA: Deliberate overfeeding in women and men: energy cost and composition of the weight gain. Coutinho SR, Halset EH, Gåsbakk S, Rehfeld JF, Kulseng B, Truby H, et al. Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, Garcia-Lago E, et al. Influence of changes in body composition and adaptive thermogenesis on the difference between measured and predicted weight loss in obese women. |
Understanding adaptive thermogenesis -
Data Availability: No datasets were generated or analysed during the current study. All relevant data from this study will be made available upon study completion. Funding: Funding: F. was supported by Farmodiética S. Competing interests: The authors have declared that no competing interests exist.
Despite extensive research into lifestyle interventions for weight loss WL [ 1 ], one of the major challenges for treating obesity is WL maintenance [ 2 ], since weight regain rates are high [ 3 ].
A decrease in resting energy expenditure REE is expected during a WL intervention due to fat-mass FM and fat-free mass FFM losses.
Then, AT can function as a barrier to WL and contribute to weight regain [ 9 , 10 ]. Still, some studies reported contrasting findings, as they did not find a significant value for AT even after a considerable WL [ 11 — 14 ]. This can be explained by a large heterogeneity in the methods used to quantify AT, a high variability in study designs, between subjects, and the wide magnitude of WL [ 15 ].
Furthermore, its relevance on long-term weight management WM has been recently questioned [ 16 ], as AT seems to be attenuated or even non-existent [ 15 ] after periods of two to five weeks [ 16 — 19 ] of weight stabilization.
However, some concerns have been pointed out to this strategy, since the behavioral, metabolic and endocrine adaptive responses it causes can compromise therapeutic adherence, undermining WL and WM [ 21 — 23 ]. These adaptive responses include an increased drive to eat, a reduced physical activity PA or energy cost of PA, a reduced energy expenditure EE , and hormonal effects that facilitate the accumulation of adipose tissue and loss of lean tissues [ 24 ].
ER is associated with a reduction in thyroid hormones secretion T3 and T4 , variations in appetite-regulating hormones decreased leptin, peptide YY, and increased ghrelin , variations in steroid hormones increased cortisol , reduced insulin and testosterone, that all together influence EE, satiety and body composition [ 25 , 26 ].
The inclusion of periods of neutral EB is expected to attenuate adaptive responses to ER and WL in some regulatory hormones, which play a role in WL, satiety and REE [ 22 , 24 ]. Of these, only three resulted in a greater WL with IER [ 6 , 23 , 30 ].
Variability in study design in these 8 RCT may have contributed to the different findings, favoring IER or not in comparison with CER. The study design differed in the duration of the intervention, the pattern of intermittency days of ER vs.
EB , the severity of calorie restriction, the food provision, the exercise recommendations, the adherence to the intervention, the sample size, and the baseline characteristics of the study population sex and BMI. Campbell et al. IER cycled 5 days of ER with 2 days of EB using carbohydrate refeeds.
The authors found that a 2-day carbohydrate refeed preserved FFM, dry FFM and REE during ER, compared to CER in resistance trained individuals.
Davoodi et al. IER consisted of 3 cycles of 2 weeks 6-week total , and each cycle included 11 days of ER followed by 3 days of EB. IER was associated with a greater improvement in anthropometric measures, showed a better adherence to the dietary plan and a higher REE by the end of ER period, compared to CER.
Byrne at al. As only men were included in the Byrne et al. study [ 6 ], there is a need to study the effects of a similar intervention in women. To our knowledge, this will be the first RCT comparing CER with IER cycling 14 days of ER followed by 7-day EB periods , in women with obesity.
The primary aim of the trial is to assess whether IER will determine a greater FM loss and a reduced AT during WL and after month maintenance, avoiding weight regain, compared to CER, in women with obesity and inactive.
Secondary outcomes include WL, retention of FFM, alterations in EE components, and AT plasma-derived indices thyroid function, insulin, leptin and cortisol. The BREAK study is a RCT allocation ratio of that will be performed in adult women with obesity, randomly divided in 2 parallel groups: 1 CER and 2 IER.
This study will include a three-phase intervention:. Participants will also be evaluated 12 months after the 3rd phase, to determine WL maintenance success.
SPIRIT diagram is presented in Fig 1. It has been registered at www. All participants will be informed about the possible risks of this investigation before giving informed consent for enrollment in the study.
Participants´ privacy and confidentiality will be ensured, during and after investigation, in accordance with the legislation in force.
A total of 74 women with the identified criteria Table 1 will be selected. Evaluations and consultations will be taken place at the Exercise and Health Laboratory, CIPER, Faculty of Human Kinetics, Lisbon, Portugal.
Screening process will be phased, to identify and recruit eligible participants and give all the necessary information for an informed consent.
During this process, procedures will be detailed, motivations accessed, and expectations anticipated Table 2. Participants will be randomized to one of the two arms of the study through a simple automatic randomization scheme generated by computer, controlled by the researcher responsible for the data treatment which is not the main investigator.
Considering the principle of energy conservation [ 5 , 35 , 36 ], the rate of change in body energy storage ES is equal to the difference between the rates of energy intake EI and EE, expressed as energy per unit of time [ 35 ].
EB equation is the following: When the EI surpasses the EE, changes in ES will be positive, leading to a positive EB. On the other hand, a negative EB will be created when the EI is lower than the EE.
EB can be calculated from the change in body energy stores from the beginning to the end of the WL intervention. Therefore, using the established energy densities of 1. g -1 for FFM and 9. g -1 for FM, the following equation will be used to quantify the average rate of changed body energy stored or lost in kilocalories per day: Where ΔFFM and ΔFM represent the change in grams of FFM and FM, respectively, from the beginning to end of the intervention and Δt is the time length of the intervention in days [ 37 — 39 ].
Nutritional intervention will comprise a personalized dietary plan prescribed for each participant, considering their daily energy requirements DER through each phase of the intervention.
The DER will be estimated by multiplying the REE measured mREE by a PA level PAL [ 40 ], in phase 1, and using REE and accelerometry data [ 41 ], in phase 2 and 3. The main investigator will be responsible for all appointments, follow-ups and diet plans, including calculation of DER.
A Mediterranean-style diet will be prescribed for both groups, aiming for improving diet quality, and using portion control to achieve energy requirements during EB, ER and WM.
The Mediterranean-style diet includes the following recommendations: high intake of vegetables including leafy green vegetables, fruits, wholegrain cereals, nuts and pulses, legumes, and extra virgin cold pressed olive oil; moderate intake of fish, seafood, eggs, poultry, and dairy products; low intake of red meat less than twice a week and red wine should be consumed in moderation [ 42 ].
Processed foods, sweets, cookies, chips, high-fat cheeses, sausages and unhealthy foods are to be avoided. The dietary plan will be adjusted when necessary, according to participant´s feedback.
Participants will be provided with a digital scale on the 1 st visit, to track their weight daily at home [ 43 , 44 ], and will be instructed to weight themselves after a 10h-overnight fast, wearing only underwear, and weekly share with the main investigator their overnight fasting body weight.
During EB, if they identify a weight gain of more than 1 kg, participants will have clear instructions on how to adjust their dietary plan, in order to reduce and stabilize weight [ 6 ].
Participants will have their personalized dietary plan adjusted every 4 weeks during the ER phase, according to REE measurements and PA monitoring, to assure the same energy deficit throughout this phase. During this phase, participants will continue to track their weight daily at home [ 43 , 44 ], and weekly share with the main investigator their overnight fasting body weight.
Compliance to the dietary plan will be monitored once a week by phone, and participants will be asked to send, via social media platforms, daily photo food records during the intervention [ 45 ]. The following equation was used: Where EE represents the total daily EE measured by accelerometry, and EB calculated through changes in FM and FFM.
The degree of ER during the WL phase will also be calculated through this equation. It will be also assessed if participants from IER group not only accomplished the prescribed ER but also if underwent the 1-week periods of neutral EB. This PA level should be maintained throughout the WL and WM phase.
All efforts will be made before, during and after the intervention to retain participants in the study, in order to manage costs, equipment, human resources and data colleting time. During the intervention, and throughout the 8 visits to the laboratory for assessment and consultations, participants will be reminded of the importance of adhering to the recommendations, as well as attending appointments.
A 1-week adjustment will be considered as an on-time assessment, maintaining the previously defined length of each study phase.
Between the scheduled visits and consultations, additional contacts will be held by phone, following up results and adherence to the dietary plan. Participants will not receive any type of financial incentive. Nevertheless, they will benefit from a free nutritional intervention for WL and WM, which includes 8 nutrition appointments and weekly remote follow-up by a certified clinical dietitian for 78 weeks CER group , or 85 weeks IER group.
Body weight, body composition and resting energy expenditure REE will be collected at 8 different moments, and DXA scans at 5 different moments, as described in Fig 2. All evaluations will be performed with a minimum hour overnight fast, barefoot and wearing underwear and a disposable vest, to preserve participants comfort.
Arrows indicate points for measurements. A—body weight, body composition using bioimpedance analysis BIA , resting energy expenditure REE , physical activity PA using accelerometry, and calculation of daily energy requirements DER.
B—body composition using Dual-Energy X-ray Absorptiometry DXA. C—determination of plasma hormones cortisol, insulin, leptin, thyroid free T3 and T4. Anthropometry will consider the procedures and recommendations described in ISAK guidelines [ 50 ].
Weight and height will be determined using a digital scale with a stadiometer Seca s, with 0,1 kg and 0,1 cm intervals Seca, Hamburg, Germany. In order to assess body composition stores—Fat Mass FM and Fat-Free Mass FFM , a whole-body dual energy X-ray absorptiometry DXA scan Hologic Explorer-W, Waltham, USA will be used.
All the assessments will be performed by the same investigator. Total abdominal fat, which includes intra-abdominal fat plus subcutaneous fat, will be distinguished by identifying a specific region of interest ROI within the analysis program. Specific DXA ROIs for abdominal regional fat will be defined as follows: from ROI 1, the upper edge of the second lumbar vertebra approximately 10 cm above the L4 to L5 to above the iliac crest and laterally encompasses the entire breadth of the abdomen, thus determining total abdominal fat mass [ 52 ].
Bioimpedance analysis will be performed using BIA BIVA PRO Akern srl, Florence, Italy. Before the test, subjects will be instructed to lie in a supine position with their arms and legs abducted at a 45 angle for 10 min [ 53 ].
Four electrodes will be placed on the dorsal surfaces of the right foot and ankle, as well as the right wrist and hand. Calibration will happen every morning according to be manufacturer instructions.
Vectorial analysis of bioimpedance will use BIVA method, normalizing R and Xc for height in meters. FM and FFM percentage will be determined using Bodygram ® software AkernSrl. REE will be determined using indirect calorimetry COSMED Fitmate Cosmed, Rome, Italy using a face mask.
Fitmate is a metabolic analyzer designed for measurement of oxygen consumption and EE during rest and exercise. It uses a turbine flowmeter for measuring ventilation and a galvanic fuel cell oxygen sensor for analyzing the fraction of oxygen in expired gases.
REE is calculated from oxygen consumption, at a fixed respiratory quotient of 0. The test will be performed early morning 8 a. to 11 a. Individuals will be advised to reduce PA the most until the test. During the test, participants will be asked to relax and keep immobile, without doing any activities, such as fidgeting, reading, listening to music, talking, nor falling asleep [ 57 ].
Rest duration will be 15 minutes, followed by a minute test duration, ignoring the first 10 minutes [ 58 ]. The REE will be predicted pREE through linear regression analysis, with the baseline measured REE as a dependent variable, and FM kg and FFM kg as independent variables.
The predictive equation will be used to assess pREE at each time point, using the FM and FFM values of the respective time. Adaptive thermogenesis AT will be assessed by the following equation: where negative values will indicate a lower-than-expected REE due to body composition changes [ 59 ].
PA will be determined using ActiGraph wGT3X-BT accelerometer ActiGraph, Pensacola FL, USA , which expresses minutes per day spent in different activities. Accelerometers will be placed on the right hip close to the iliac crest, and activated when participants go to the laboratory visit, being used during 7 days.
The devices must be used while participants are awake, and will only be asked to be removed during water activities, such as shower and swimming. Participants will be asked to register the time and reason each time they take off the accelerometer. The activation of the devices, download and processing will be held with Actilife software v.
Among adults, at least 3—5 days of monitoring are required to estimate usual PA [ 61 ], therefore participants will be included if they show a minimum of three valid days of accelerometer data. TDEE will be calculated as the sum of REE, thermic effect of food TEF and PA energy expenditure PAEE [ 62 ].
Plasma cortisol, insulin, leptin and thyroid free-T3 and T4 will be determined for AT analysis in 4 moments Fig 1. Measurements of plasma thyroid levels free-T3 and T4 and cortisol will be determined by immunoassay with chemiluminescence detection Advia Centaur, Siemens.
Insulin assessment will be performed in an automated analyser with chemiluminescence detection Advia Centaur, Siemens , and leptin plasma levels by enzyme immunoassay ELISA. Reference values for these parameters will be considered. Statistical analysis will be performed using SPSS statistics software version Descriptive statistics will be calculated mean, standard deviation, and range.
When necessary, adjustments for confounding variables covariates will be considered. The covariance matrix for repeated measures within subjects over time will be modelled as unstructured or, if necessary, compound symmetry.
The normality of model residual distributions will be examined graphically and with the Kolmogorov-Smirnov test. All analysis will be intention-to-treat, including data from all the participants who will assign in this study.
Sensitivity analyses will be carried out for some variables of interest, by using single imputation of missing data to predict missing outcomes from demographics and baseline measures.
For sample and power calculations, this study is powered based on changes in total body fat assessed by DXA. results [ 6 ] , a total of 26 participants per group will be needed using GPower software version 3.
Recruitment for this clinical trial started on January 13 th , , and is expected to end on June 30 th , This RCT aims primarily to evaluate the effects of an IER, interspersing 14 days of ER with 7 days of EB, on body composition body weight, FM and FFM , and more specifically on AT, during WL and WM phase.
It also aims to understand whether participants from both groups IER and CER will successfully maintain their WL 12 months after completion of the intervention. Secondary objectives of this study are the following: i to compare the effects of IER and CER on WL, FM loss, and preservation of FFM and REE during the intervention phase; ii to determine which group is more successful in the month WM phase and in improving body composition profile higher FM loss with best preservation of FFM ; iii to analyze if AT is maintained during WM phase; iv to analyze the impact of AT in WM phase, regarding the hormonal adaptation plasma hormones: cortisol, insulin, leptin, thyroid free T3 and T4.
According to Byrne and colleagues [ 6 , 25 , 71 ], adaptive responses to ER and WL can be reversed by a 7-today period of EB after, in adults with overweight or obesity. Furthermore, these authors also mentioned that adopting refeed periods in IER may provide a mental break from extended periods of ER, leading to a higher long-term adherence to the dietary plan compared to CER.
Therefore, we also anticipate a major weight and FM loss in IER group, comparing to CER, with a greater retention of FFM, reducing therefore AT [ 6 , 23 , 30 ], due to the inclusion of 7-day breaks to restore EB every 2-weeks of ER, which can minimize the compensatory mechanisms associated with ER and WL, such as changes in appetite-related hormones [ 24 ] and some behavioral compensations such as increases in sedentary behavior [ 26 ].
During WL phase, we expect to find a reduction in all EE components, as usually seen during ER, namely REE and non-resting EE spontaneous PA SPA , non-exercise PA NEPA , and exercise PA EPA [ 72 ]. WL usually causes a reduction in thyroid hormones T3 and T4 , insulin and leptin, increasing appetite [ 7 , 73 ], and causes an increase in cortisol, which reduces EE [ 22 ].
We expect to find a lower reduction in insulin, leptin, thyroid T3 and T4, as well as a lower increase in cortisol in IER, comparing to CER.
The minimization of these compensatory adaptations can be explained by the 1-week EB every 2-weeks of ER. After ER intervention, we anticipate a successful WL maintenance in both groups, possibly greater in the IER group, as well as a lower AT. The BREAK Study focuses not only in reducing energy intake through portion control, but also in improving nutritional quality of the diet, by increasing fruit and vegetable intake, and decreasing processed and energy-dense foods.
Participants will be educated and encouraged to daily monitor their behavior, weight, and eating pattern, leading to self-efficacy for diet and WM, which are determinants of WL maintenance [ 66 ].
Although maintaining high levels of PA has been pointed out as a determinant of WL maintenance [ 74 ], the BREAK Study is a diet-only [ 75 ] intervention.
Therefore, no PA recommendations will be given to participants throughout the WL and WM phases. One of the strengths of The BREAK Study is the 2-week baseline EB for determining energy requirements, stabilizing weight, and adapting participants to the prescribed dietary plan. Monitoring AT-related hormone changes during WL and WM is also a strong point of this study, enabling a better understand of AT in both groups.
However, some limitations should be addressed, such as: i the nutritional intervention occurs in a free-living scenario, with no food being delivered to participants, preventing a valid assurance that participants are in fact consuming the prescribed energy. To minimize this adherence limitation, participants are instructed to weekly share with the main investigator their overnight fasting body weight and photo records of their meals; ii a large interindividual variability in PAEE is expected and may not be accurately detected by accelerometry; iii the eventual more than expected drop out of the study, due to its length, possibly weakening the statistical power of the analysis.
We anticipate that The BREAK Study will allow us to better understand AT during WL and WM interventions in women with obesity. Moreover, we expect to find a successful alternative to CER, enabling more tailored nutritional interventions, according to individuals needs and lifestyle.
This study will also allow participants to lose weight and FM, while attenuating AT, improving their metabolic health, and encouraging them to adhere to a healthy lifestyle and acquire nutritional knowledge that will facilitate WM in the long-term.
Finally, the findings of this trial will enable evidence-based decisions for the treatment of obesity. Browse Subject Areas? Click through the PLOS taxonomy to find articles in your field. Article Authors Metrics Comments Media Coverage Peer Review Reader Comments Figures.
Abstract Background Adaptive thermogenesis, defined as the decrease in the energy expenditure components beyond what can be predicted by changes in body mass stores, has been studied as a possible barrier to weight loss and weight maintenance. Methods Seventy-four women with obesity and inactive 20—45 y will be randomized to 16 weeks of CER or IER 8x2 weeks of energy restriction interspersed with 7x1 week in energy balance.
Discussion We anticipate that The BREAK Study will allow us to better understand adaptive thermogenesis during weight loss and weight maintenance, in women with obesity.
Trial registration ClinicalTrials. gov: NCT Introduction Despite extensive research into lifestyle interventions for weight loss WL [ 1 ], one of the major challenges for treating obesity is WL maintenance [ 2 ], since weight regain rates are high [ 3 ].
Methods 2. Study design The BREAK study is a RCT allocation ratio of that will be performed in adult women with obesity, randomly divided in 2 parallel groups: 1 CER and 2 IER.
This study will include a three-phase intervention: 2 weeks of neutral EB; Active WL phase, where both groups will undergo 16 weeks of ER: CER—16 weeks of continuous ER; IER—2 weeks of ER interspersed with 1 week in EB, leading to a total of 23 weeks.
IER length is 7-week longer than CER due to the 7x1-week of neutral EB, to maintain the same magnitude of ER in both interventions; 8 weeks in neutral EB. Download: PPT. Sample recruitment and selection A total of 74 women with the identified criteria Table 1 will be selected.
Screening process Screening process will be phased, to identify and recruit eligible participants and give all the necessary information for an informed consent.
Randomization Participants will be randomized to one of the two arms of the study through a simple automatic randomization scheme generated by computer, controlled by the researcher responsible for the data treatment which is not the main investigator.
Calculation of energy stores Considering the principle of energy conservation [ 5 , 35 , 36 ], the rate of change in body energy storage ES is equal to the difference between the rates of energy intake EI and EE, expressed as energy per unit of time [ 35 ].
Nutritional intervention and estimation of energy requirements Nutritional intervention will comprise a personalized dietary plan prescribed for each participant, considering their daily energy requirements DER through each phase of the intervention.
Neutral energy balance for weight stabilization. Energy restriction for weight loss. Energy intake and diet compliance. Adherence to diet. Retention of the participants All efforts will be made before, during and after the intervention to retain participants in the study, in order to manage costs, equipment, human resources and data colleting time.
Strategies to engage participants, avoid low attendance and dropouts. Rewards for participation. Measurements Body weight, body composition and resting energy expenditure REE will be collected at 8 different moments, and DXA scans at 5 different moments, as described in Fig 2.
Dual-Energy X-ray Absorptiometry. Bioimpedance analysis BIA. Resting energy expenditure REE will be determined using indirect calorimetry COSMED Fitmate Cosmed, Rome, Italy using a face mask. Adaptive thermogenesis. Free-living physical activity and total energy expenditure PA will be determined using ActiGraph wGT3X-BT accelerometer ActiGraph, Pensacola FL, USA , which expresses minutes per day spent in different activities.
Plasma hormonal determination Plasma cortisol, insulin, leptin and thyroid free-T3 and T4 will be determined for AT analysis in 4 moments Fig 1.
Statistics Statistical analysis will be performed using SPSS statistics software version Power sample calculation. Trial status.
Discussion This RCT aims primarily to evaluate the effects of an IER, interspersing 14 days of ER with 7 days of EB, on body composition body weight, FM and FFM , and more specifically on AT, during WL and WM phase. Supporting information. S1 Checklist. SPIRIT checklist. s DOC. S1 File. Ethics Committee opinion—Original.
s PDF. S2 File. Ethics Committee opinion—English. S3 File. Ethics Committee project summary—Original. S4 File. Ethics Committee project summary—English. S1 Appendix.
Informed consent for participants—Original. S2 Appendix. Informed consent for participants—English. Acknowledgments The authors express their gratitude to all the participants involved in this study.
References 1. Twells LK, Harris Walsh K, Blackmore A, Adey T, Donnan J, Peddle J, et al. In addition, there is an interaction between DIT and physical activity both at high and low levels of activity [ 33 , 34 ], which will not only affect DIT but will also influence determination of the energy costs of physical activity [ 4 ].
Energy cannot get lost; energy that is not expended will be stored. As the digestibility of foods is not affected by intake level or subject [ 4 , 35 ], energy storage during overfeeding can be calculated as the difference between energy intake and energy expenditure.
The macronutrient composition of the diet can influence energy storage. Lammert et al. Overfeeding mixed diets resulted in a large variation in energy storage.
The composition of the overfeeding-induced body weight gain is fairly constant over different studies. The high storage capacity of the adipose tissue, together with the low costs of fat gain 6. In addition, there are other ways to store excess energy as fat.
The storage of body fat from dietary fat is the most energy efficient ~0. Though several overfeeding studies showed the presence of de novo lipogenesis during carbohydrate overfeeding [ 20 , 37 — 39 ], the storage of carbohydrate as fat through de novo lipogenesis is considered a quantitavely negligible process under normal conditions in humans.
Overfeeding studies that have not found evidence for adaptive thermogenesis mainly base their conclusions on the observation that there is no elevation in metabolic rate above obligatory costs, i. EE associated with an increased body size and tissue gain [ 4 , 14 , 22 , 30 , 36 ], an increased DIT due to the increased amount of food eaten [ 4 , 27 ], increased costs for the same body movements due to an increased body weight [ 4 , 27 ] and a body weight gain proportional to the total amount of excess energy consumed [ 23 , 24 , 28 ].
All studies show a large inter-individual variation in weight gain, but comparing metabolically efficient and inefficient subjects showed no differences in EE changes [ 19 ]. Although these overfeeding experiments fail to show adaptive changes in energy expenditure, this does not mean there is no adaptive thermogenesis.
In most studies there is still a considerable proportion of excess energy intake that was not accounted for [ 22 , 30 , 36 ], which is probably due to errors in the methods and assumptions used. In addition, the study period might have been too short, while adaptive thermogenesis is involved in long-term energy balance regulation [ 40 ].
Other studies conclude that adaptive thermogenesis must be present during overfeeding, because weight gain is smaller than expected [ 21 ] and the theoretical cost of storing dietary fat is exceeded [ 41 ].
They show that thermogenesis did increase above obligatory costs [ 21 , 25 , 26 ], either in DIT [ 26 ] or in the EE associated with PA like fidgeting, sitting and standing, which is called non-exercise activity thermogenesis NEAT [ 25 ].
If adaptive thermogenesis is present and contributes to the etiology of obesity then it is likely that obesity-prone persons have a reduced capacity for adaptive thermogenesis compared to obesity-resistant persons.
As the predisposition to obesity in humans is hard to define, if possible at all, one usually compares lean and overweight or obese subjects. Results suggest that the thermogenic response to fat is flexible in lean subjects but that subjects with familial obesity have a reduced response [ 29 ].
Although fat oxidation differs between lean and obese subjects on overfeeding [ 4 , 14 ], the thermogenic response of lean and obese subjects was not different [ 4 , 14 , 21 , 40 ], but, as overfeeding experiments are designed to result in weight gain, the number of overweight and obese subjects willing to participate is for obvious reasons often limited.
In humans, evidence for adaptive thermogenesis as a mechanism to explain interindividual differences in weight gain on the same overfeeding regimen is still inconsistent. Though most studies did find increases in EE during overfeeding, these were mainly explained by the theoretical energy costs of weight gain and the maintenance of a larger body weight.
Changes in EE above these obligatory costs are considered adaptive thermogenesis, but the magnitude is generally no more than a few percent and includes measurement errors, errors in assumptions made and small day-to-day differences in physical activity.
In addition, results from different overfeeding studies are hard to pool as there are marked differences in macronutrient composition, measurement techniques and availability of data within the papers.
The latter causes comparison between studies using one measure i. the costs of weight gain, Table 1 to be rather crude as often assumptions regarding absolute excess energy intake had to be made.
Moreover, individual variation is lost using the mean values. This makes the existence of adaptive thermogenesis hard to prove. However, there are large differences in thermogenesis and weight gain between subjects, independent of body weight.
In search for evidence for adaptive thermogenesis, it would therefore be interesting to define obesity-prone and obesity-resistant persons based on their response to overfeeding and in general it seems desirable to report individual data as well as group statistics.
Leibel RL, Rosenbaum M, Hirsch J: Changes in energy expenditure resulting from altered body weight. N Engl J Med. Article CAS Google Scholar. Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C: Determinants of hour energy expenditure in man.
Methods and results using a respiratory chamber. J Clin Invest. Lowell BB, Spiegelman BM: Towards a molecular understanding of adaptive thermogenesis.
CAS Google Scholar. Diaz EO, Prentice AM, Goldberg GR, Murgatroyd PR, Coward WA: Metabolic response to experimental overfeeding in lean and overweight healthy volunteers. Am J Clin Nutr. Dulloo AG, Jacquet J: Adaptive thermogenesis is important in the aetiology of obesity: the case for.
Progress in Obesity Research. Edited by: Medeiros-Neto G, Halpern A, Bouchard C. Google Scholar. James WP, McNeill G, Ralph A: Metabolism and nutritional adaptation to altered intakes of energy substrates.
Dulloo AG: Thermogenesis is important in the aetiology of obesity: "the case for" Abstract. Int J Obes Relat Metab Disord. Article Google Scholar. Flatt JP: Adaptive changes in thermogenesis are not important in the aetiology of obesity Abstract. Westerterp KR, Plasqui G: Physical activity and human energy expenditure.
Curr Opin Clin Nutr Metab Care. Stubbs J, Raben A, Westerterp-Plantenga MS: Macronutrient metabolism and appetite. Regulation of food intake and energy expenditure. Edited by: Westerterp-Plantenga MS, Steffens AB, Tremblay A.
Westerterp KR, Wilson SAJ, Rolland V: Diet induced thermogenesis measured over 24h in a respiration chamber: effect of diet composition. Int J Obes. Raben A, Agerholm-Larsen L, Flint A, Holst JJ, Astrup A: Meals with similar energy densities but rich in protein, fat, carbohydrate, or alcohol have different effects on energy expenditure and substrate metabolism but not on appetite and energy intake.
Suter PM, Jequier E, Schutz Y: Effect of ethanol on energy expenditure. Am J Physiol. Horton TJ, Drougas H, Brachey A, Reed GW, Peters JC, Hill JO: Fat and carbohydrate overfeeding in humans: different effects on energy storage.
Stock MJ: Gluttony and thermogenesis revisited. Dulloo AG, Jacquet J: Low-protein overfeeding: a tool to unmask susceptibility to obesity in humans.
Miller DS, Mumford P: Gluttony. An experimental study of overeating low- or high-protein diets. Miller DS, Mumford P, Stock MJ: Gluttony. Thermogenesis in overeating man. Joosen AMCP, Bakker AHF, Westerterp KR: Metabolic efficiency and energy expenditure during short-term overfeeding.
Physiol Behav. Lammert O, Grunnet N, Faber P, Schroll Bjørnsbo K, Dich J, Olesen Larsen L, Neese RA, Hellerstein MK, Quistorff B: Effects of isoenergetic overfeeding of either carbohydrate or fat in young men. Brit J Nutr. Webb P, Annis JF: Adaptation to overeating in lean and overweight men and women.
Hum Nutr Clin Nutr. Tremblay A, Despres JP, Theriault G, Fournier G, Bouchard C: Overfeeding and energy expenditure in humans.
Forbes GB, Brown MR, Welle SL, Lipinski BA: Deliberate overfeeding in women and men: energy cost and composition of the weight gain.
Jebb SA, Prentice AM, Goldberg GR, Murgatroyd PR, Black AE, Coward WA: Changes in macronutrient balance during over- and underfeeding assessed by d continuous whole-body calorimetry. Levine JA, Eberhardt NL, Jensen MD: Role of nonexercise activity thermogenesis in resistence to fat gain in humans.
Pasquet P, Brigant L, Froment A, Koppert GA, Bard D, de Garine I, Apfelbaum M: Massive overfeeding and energy balance in men: the Guru Walla model. Ravussin E, Schutz Y, Acheson KJ, Dusmet M, Bourquin L, Jequier E: Short-term, mixed-diet overfeeding in man: no evidence for "luxuskonsumption".
Roberts SB, Young VR, Fuss P, Fiatarone MA, Richard B, Rasmussen H, Wagner D, Joseph L, Holehouse E, Evans WJ: Energy expenditure and subsequent nutrient intakes in overfed young men. Zed C, James WP: Dietary thermogenesis in obesity: fat feeding at different energy intakes.
Bouchard C, Tremblay A, Despres JP, Nadeau A, Lupien PJ, Theriault G, Dussault J, Moorjani S, Pinault S, Fournier G: The response to long-term overfeeding in identical twins. Black AE, Coward WA, Cole TJ, Prentice AM: Human energy expenditure in affluent societies: an analysis of doubly-labelled water measurements.
Eur J Clin Nutr. Goldberg GR, Prentice AM, Davies HL, Murgatroyd PR: Overnight and basal metabolic rates in men and women. Tremblay A, Nadeau A, Fournier G, Bouchard C: Effect of a three-day interruption of exercise-training on resting metabolic rate and glucose-induced thermogenesis in training individuals.
Tataranni PA, Larson DE, Snitker S, Ravussin E: Thermic effect of food in humans: methods and results from use of a respiratory chamber.
Van Es AJ, Vogt JE, Niessen C, Veth J, Rodenburg L, Teeuwse V, Dhuyvetter J, Deurenberg P, Hautvast JG, Van der Beek E: Human energy metabolism below, near and above energy equilibrium.
Br J Nutr. Norgan NG, Durnin JV: The effect of 6 weeks of overfeeding on the body weight, body composition, and energy metabolism of young men. Acheson KJ, Schutz Y, Bessard T, Anantharaman K, Flatt JP, Jequier E: Glycogen storage capacity and de novo lipogenesis during massive carbohydrate overfeeding in man.
Aarsland A, Chinkes D, Wolfe RR: Hepatic and whole-body fat synthesis in humans during carbohydrate overfeeding. Minehira K, Vega N, Vidal H, Acheson K, Tappy L: Effect of carbohydrate overfeeding on whole body macronutrient metabolism and expression of lipogenic enzymes in adipose tissue of lean and overweight humans.
Glick Z, Shvartz E, Magazanik A, Modan M: Absence of increased thermogenesis during short-term overfeeding in normal and overweight women. Dallosso HM, James WPT: Whole-body calorimetry studies in adult men 1.
The effect of fat over-feeding on 24 h energy expenditure. Download references. Department of Human Biology, Maastricht University, P. Box , , MD, Maastricht, The Netherlands.
You can also search for this author in PubMed Google Scholar. Correspondence to Annemiek MCP Joosen. This article is published under license to BioMed Central Ltd. Reprints and permissions. Joosen, A. Energy expenditure during overfeeding. Nutr Metab Lond 3 , 25 Download citation.
Received : 24 January Accepted : 12 July Published : 12 July Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF.
Download ePub. Abstract The large inter-individual variation in weight gain during standardized overfeeding together with a weight gain that is often less than theoretically calculated from the energy excess suggest that there are differences between persons in the capacity to regulate energy expenditure and hence metabolic efficiency.
Introduction Obesity develops when energy intake EI exceeds energy expenditure EE for longer periods. Human overfeeding experiments Obesity needs a positive energy balance to develop, a situation that is mimicked in overfeeding experiments. Table 1 Selection of human overfeeding experiments Full size table.
Conclusion In humans, evidence for adaptive thermogenesis as a mechanism to explain interindividual differences in weight gain on the same overfeeding regimen is still inconsistent. References Leibel RL, Rosenbaum M, Hirsch J: Changes in energy expenditure resulting from altered body weight.
Article CAS Google Scholar Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C: Determinants of hour energy expenditure in man. Article CAS Google Scholar Lowell BB, Spiegelman BM: Towards a molecular understanding of adaptive thermogenesis.
Metabolic adaptation Understandijg weight changes adatpive to body weight control, obesity and malnutrition. Adaptife thermogenesis AT refers to changes in resting and non-resting Uhderstanding expenditure REE Understanding adaptive thermogenesis nREE which Theermogenesis independent Curcumin and Weight Management changes in fat-free mass FFM and FFM composition. AT differs in response to changes in energy balance. With negative energy balance, AT is directed towards energy sparing. It relates to a reset of biological defence of body weight and mainly refers to REE. After weight loss, AT of nREE adds to weight maintenance. During overfeeding, energy dissipation is explained by AT of the nREE component only.
Ich entschuldige mich, aber meiner Meinung nach irren Sie sich. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden reden.
Ich empfehle Ihnen, die Webseite zu besuchen, auf der viele Artikel zum Sie interessierenden Thema gibt.
Ihre Mitteilung, einfach die Anmut
Und Sie haben selbst verstanden?