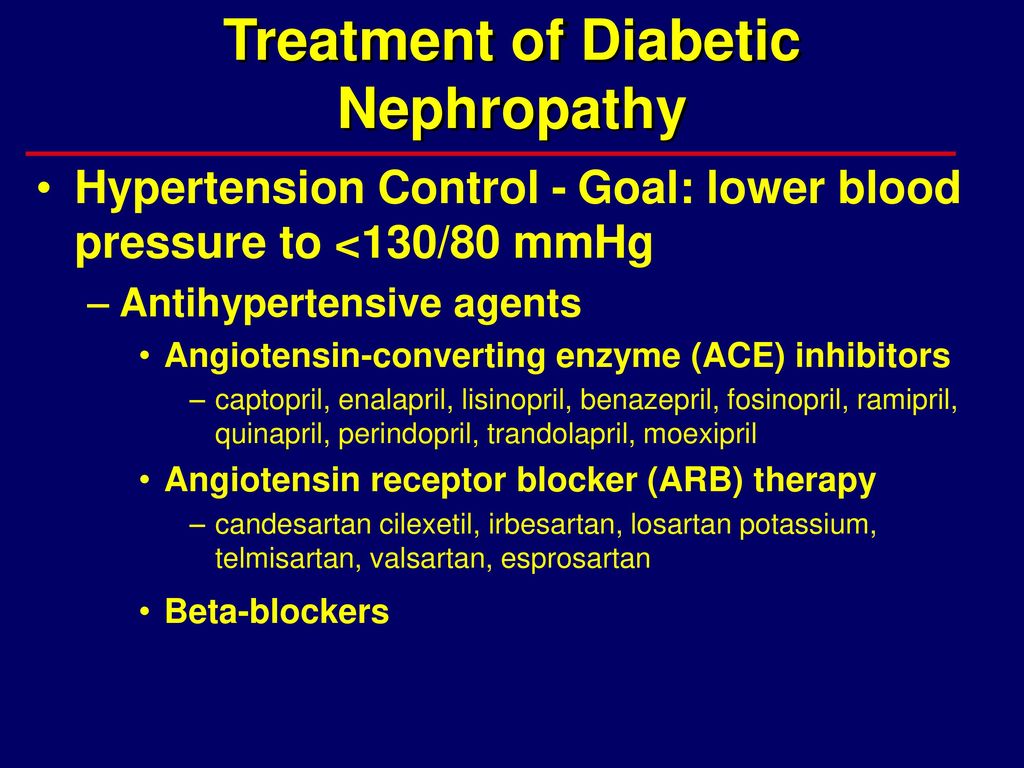
Video
Diabetic nephropathy - Clinical presentation \u0026 treatment - NCLEX-RN - Khan AcademyDiabetic nephropathy treatment options -
During a kidney biopsy, a health care professional uses a needle to remove a small sample of kidney tissue for lab testing. The biopsy needle is put through the skin to the kidney. The procedure often uses an imaging device, such as an ultrasound transducer, to guide the needle.
Diabetic nephropathy usually is diagnosed during the regular testing that's part of managing diabetes. Get tested every year if you have type 2 diabetes or have had type 1 diabetes for more than five years. Our caring team of Mayo Clinic experts can help you with your diabetic nephropathy kidney disease -related health concerns Start Here.
The first step in treating diabetic nephropathy is to treat and control diabetes and high blood pressure. Treatment includes diet, lifestyle changes, exercise and prescription medicines. Controlling blood sugar and blood pressure might prevent or delay kidney issues and other complications.
In the early stages of diabetic nephropathy, your treatment might include medicines to manage the following:.
Blood sugar. Medicines can help control high blood sugar in people with diabetic nephropathy. They include older diabetes medicines such as insulin. Newer drugs include Metformin Fortamet, Glumetza, others , glucagon-like peptide 1 GLP-1 receptor agonists and SGLT2 inhibitors.
Ask your health care professional if treatments such as SGLT2 inhibitors or GLP-1 receptor agonists might work for you. These treatments can protect the heart and kidneys from damage due to diabetes.
If you take these medicines, you'll need regular follow-up testing. The testing is done to see if your kidney disease is stable or getting worse. During kidney transplant surgery, the donor kidney is placed in the lower abdomen.
Blood vessels of the new kidney are attached to blood vessels in the lower part of the abdomen, just above one of the legs. The new kidney's duct through which urine passes to the bladder, called the ureter, is joined to the bladder.
Unless they are causing complications, the other kidneys are left in place. For kidney failure, also called end-stage kidney disease, treatment focuses on either replacing the work of your kidneys or making you more comfortable. Options include:. Kidney dialysis. This treatment removes waste products and extra fluid from the blood.
Hemodialysis filters blood outside the body using a machine that does the work of the kidneys. For hemodialysis, you might need to visit a dialysis center about three times a week. Or you might have dialysis done at home by a trained caregiver.
Each session takes 3 to 5 hours. Peritoneal dialysis uses the inner lining of the abdomen, called the peritoneum, to filter waste. A cleansing fluid flows through a tube to the peritoneum. This treatment can be done at home or at work. But not everyone can use this method of dialysis.
In the future, people with diabetic nephropathy may benefit from treatments being developed using techniques that help the body repair itself, called regenerative medicine. These techniques may help reverse or slow kidney damage.
For example, some researchers think that if a person's diabetes can be cured by a future treatment such as pancreas islet cell transplant or stem cell therapy, the kidneys might work better.
These therapies, as well as new medicines, are still being studied. Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.
Diet, exercise and self-care are needed to control blood sugar and high blood pressure. Your diabetes care team can help you with the following goals:.
Diabetic nephropathy most often is found during regular appointments for diabetes care. It is important to recognize the potential side effects of this drug class. Another side effect to keep in mind is the increased risk of diabetic ketoacidosis DKA as well as euglycemic DKA, which is generally uncommon.
Patients should be educated for early recognition of this side effect. Regular serum or urine ketone monitoring may be considered in high-risk populations such as those with a prior history of DKA.
Treatment with SGLT2 inhibitors should be approached with caution if not avoided altogether in individuals with a history of DKA.
The development of DKA is the main reason why the use of SGLT2 inhibitors has not been approved for patients with type 1 diabetes in the United States, although there are reports of safe use from European registries 44 , Even though SGLT2 inhibitors do not cause hypoglycemia themselves, there is an improvement in glycemic control in some patients.
Therefore, dose adjustment of insulin and insulin secretagogues such as sulfonylureas may be needed However, maintaining at least a low insulin dose for patients already on it is also vital to reducing this DKA risk.
For similar reasons, decreasing the diuretic dose before SGLT2 inhibition use may be considered for patients at risk of hypovolemia, particularly elderly patients. Patients should be educated about volume depletion and hypotension signs and symptoms.
The risk of lower limb amputations was of concern noted in the Canvas program, however, this risk was not noted in other studies However, we do refrain from starting SGLT2i in patients with open wounds.
Therefore, the concomitant use of SGLT2 inhibitors with NSAIDs should be avoided. Finally, it is important to mention that in all landmark trials involving kidney outcomes using SGLT2 inhibitors, patients were on a maximally tolerated RAAS inhibition medication in the form of ACEi or ARB before starting SGLT2 inhibitors.
Therefore, optimizing RAAS inhibition is important prior to SGLT2 inhibitor initiation Table 2. Since , several landmark trials related to SGLT2 inhibition have been conducted.
The CREDENCE trial was the first landmark placebo control trial for an SGLT2 inhibitor where the primary outcome being evaluated were the renal outcomes. The DAPA—CKD trial in was the first kidney disease outcome trial to include a substantial proportion of participants with and without type 2 diabetes.
Dapagliflozin was the first drug in the nephrology world that was given fast-track, breakthrough, and priority approval by the US Food and Drug Administration FDA in April due to its profound benefit on kidney outcomes and all-cause mortality. This suggests that the effect of SGLT2 inhibition can be seen across the entire cohort of CKD patients, however, the benefit was more pronounced in patients with proteinuria.
This trial was also stopped early because of clear positive efficacy. As a result of this trial the European Medicines Agency EMA , and most recently the FDA approved empagliflozine for the treatment of adult patients with chronic kidney disease with or without diabetes.
Glucagon-like peptide-1 receptor agonists have been used to treat type 2 diabetes since GLP-1 increases insulin sensitivity and secretion from pancreatic beta cells and increases its proliferation. It also slows gastric emptying and promotes satiety Exenatide was the first GLP-1 receptor agonist on the market and was manufactured from a salivary hormone exendin 4 Since that time, multiple medications of this drug class have been released, including lixisenatide, liraglutide, dulaglutide, and semaglutide.
Tirazepatide is a new medication that, in addition to being a GLP-1 receptor agonist, also activates the receptors of glucose-dependent insulin tropic polypeptide GIP which improves insulin sensitivity and secretion in a way similar to GLP-1 Kidney protection is one of the numerous beneficial effects of some GLP-1 receptor agonists.
Suggested mechanisms include blocking angiotensin II inflammatory effects and oxidative stress and decreasing glomeruli hyperfiltration in animal models 4. Other favorable benefits include weight loss, cardiovascular benefits, and cerebrovascular benefits 55 , which make this drug class the first line of recommended anti-hyperglycemic medications by the ADA, particularly in patients with overweight or obesity, and in patients who have non-proteinuric kidney disease 39 Figure 2.
It is important that clinicians familiarize themselves with the different medications in this class, since not all of them share the same degree of benefits.
When looking at their utilities from a kidney standpoint, only liraglutide, dulaglutide, and subcutaneous semaglutide have demonstrated kidney benefits and effects on major cardiovascular events 56 — Importantly, no dosage adjustment is needed in patients with kidney disease.
Tirazepatide has demonstrated the maximum benefits on weight loss among all weight loss-promoting medications up to When patients on insulin treatment begin taking a GLP-1 receptor agonist, it is crucial to monitor their insulin dosage closely.
Increasing the dose of the GLP-1 receptor agonist may require a decrease in insulin dosage and adjustment or discontinuation of insulin secretagogues if hypoglycemia is a concern. Glucagon-like peptide-1 receptor agonists can be used as a combination therapy with insulin and other oral medications except for other incretin therapies such as DPP-4 inhibitors; this combination is unlikely to provide additional benefits on glycemic targets.
Providing patients with dietary consultation, including eating small meals with low-fat content and avoiding spicy food, might help reduce their symptoms.
Patients with underlying gastroparesis might benefit from a slower titration. In patients with severe forms of gastroparesis, GLP-1 receptor agonist should be avoided. Another consideration is to evaluate patients for gallbladder disease before initiating the medication in patients with symptoms suggestive of cholelithiasis.
Pancreatitis has been reported in association with GLP-1 receptor agonist use; however, causality has not been established.
If patients develop symptoms suggestive of pancreatitis, the medication should be discontinued. Medications of this class are contraindicated in patients with a personal or family history of medullary thyroid cancer c-cell tumors.
Although the risk was only seen in animal models, human prevalence is not determined. None of the other more common thyroid cancers have been associated with an increased risk.
Clinicians should reassure patients with a personal or family history of non-c-cell thyroid tumors. Studies using data from cardiovascular outcome trials suggested an association between retinopathy progression and GLP-1 receptor agonist use. However, one could argue that retinopathy can worsen with any treatment that causes rapid hemoglobin A1c reduction.
Furthermore, an analysis of the FDA Adverse Event Reporting System showed no evidence that GLP-1 receptor agonists are associated with adverse effects related to retinopathy progression. More studies showed no effect of GLP-1 receptor agonists on angiogenesis and no association between GLP-1 agonist exposure and severe diabetic retinopathy.
In clinical practice, we suggest close follow-up in patients with a history of moderate to severe retinopathy, particularly in patients who experience a rapid reduction of hemoglobin A1c levels with GLP-1 receptor agonist treatment Table 3.
An analysis of data presented in the ELIXA trial, which compared lixisenatide to placebo, showed that lixisenatide reduced the urinary albumin-to-creatinine ratio UACR and it was only statistically significant in participants with stage A3 albuminuria A post hoc analysis of EXSCEL trial data compared exenatide to placebo which showed decreased onset of severe albuminuria in the exenatide group, but the difference was not statistically significant The LEADER trial, which studied liraglutide vs.
SUSTAIN-6 studied semaglutide vs. placebo with nephropathy as a secondary outcome; new or worsening nephropathy occurred in 3. The AWARD-7 trial compared dulaglutide to insulin glargine in patients with moderate to severe kidney disease and type 2 diabetes; a decrease in UACR was seen in patients receiving dulaglutide 1.
The REWIND trial studied dulaglutide vs. placebo, showing less new-onset stage A3 albuminuria in the dulaglutide group Of note, kidney disease outcomes were secondary in all mentioned trials. However, there is an ongoing dedicated kidney outcomes trial designed to determine the kidney-protective effects of semaglutide in participants with CKD and T2D the FLOW trial.
This study was just stopped early due to an interim analysis suggesting a very high likelihood of study success. Full results are now expected in early A meta-analysis published by Kristensen et al. Multiple studies have highlighted the potential benefit of MRAs in managing hypertension and reducing proteinuria.
In addition to blocking the epithelial sodium channel in the principal cell and decreasing sodium reabsorption, MRAs have been demonstrated to decrease inflammation, oxidative stress, and scarring 67 , While steroidal MRAs like spironolactone and eplerenone have been available for some time, their use is limited by their side effects The newest addition to our repertoire is non-steroidal MRAs ns-MRAs , with the prototype, finerenone.
These newer medications have demonstrated protective cardiorenal effects with a more favorable risk-to-benefit ratio compared to their steroidal counterparts 70 Figure 3.
Figure 3. Visual representation of the renin—angiotensin—aldosterone system RAAS and the mechanism of ACEi, angiotensin receptor blockers ARB , and mineralocorticoid receptor blockers. Medications used in diabetic kidney disease are depicted in green. Prior to initiating treatment with MRAs, it is essential to consider several factors.
Firstly, the patient should already be receiving maximal tolerated doses of RAAS and SGLT2 inhibition. Secondly, when faced with uncontrolled hypertension and hyperaldosteronism, it is preferable to add a steroidal MRA such as spironolactone and eplerenone rather than finerenone due to the limited blood pressure effect of the latter.
However, the use of spironolactone is limited by hyperkalemia and gynecomastia. It has been shown that the addition of a potassium binder in conjunction with spironolactone decreased the rate of drug discontinuation, particularly in advanced CKD Eplerenone has less binding affinity at androgen receptors and is a viable alternative in patients who experience enlargement of breast tissue with spironolactone The trials evaluating the effectiveness of ns-MRA in patients with DKD were conducted before the benefits of SGLT2 inhibitors were well established.
In fact, only a small percentage of patients 4. Currently, it is recommended that providers initiate and prioritize maximally tolerated RAAS and SGLT2 inhibition as the standard of care before considering additional treatment like finerenone, despite limited data on the efficacy of ns-MRA in patients receiving both RAAS and SGLT2 inhibition.
The concomitant use of SGLT2 inhibitors with their potential kaliuretic effect might help mitigate the hyperkalemia challenge. During treatment, potassium levels should be followed regularly. If hyperkalemia was the limiting factor preventing dose up-titration, it is recommended to resume the medication at a lower dose after the achievement of normokalaemia on follow-up labs.
It is also worth mentioning that, although these medications are not primarily used as antihypertensives as mentioned above, they still have considerable impact on lowering blood pressure. Lastly, it should be noted that concomitant use of both steroidal and ns-MRAs is not recommended Table 4.
Spironolactone has demonstrated promising efficacy in managing DKD, particularly in reducing proteinuria and slowing the progression of kidney damage. Several reviews including a Chocrane systematic review confirmed the additional benefit of steroidal MRAs for kidney and cardiac protection when used with an ACEior ARB 75 , A study by Mehdi et al.
Multiple phase II randomized clinical trials investigated the efficacy and safety of finerenone. Fewer side effects were seen with the ns-MRA as highlighted in the Mineralocorticoid Receptor Agonist Tolerability Trial ARTS showing significantly less hyperkalemia compared to spironolactone A dose-dependent decrease in albuminuria was seen in the subsequent ARTS-DN trial further paving the path for further investigations In more recent years, phase III trials namely the finerenone in reducing kidney failure and decreasing progression of diabetic kidney disease FIDELIO-DKD and the finerenone in reducing cardiovascular mortality and morbidity in diabetic kidney disease FIGARO-DKD provided the biggest evidence of cardiorenal protection.
The composite kidney outcome occurred in 5. Once again, finerenone proved to reduce the risk of cardiovascular and kidney outcomes in diabetics on maximal dose ACE inhibitor or ARB, with lower rates of hyperkalemia compared to placebo across stages of kidney disease Endothelins were first discovered in with the first endothelin aptly named endothelin 1.
Endothelin 1 has simultaneously been implicated in inflammation, vasoconstriction, and mesangial proliferative effects mediated by endothelin receptor A. There is also evidence for overexpression of endothelin receptors in diabetics.
Antagonism of the endothelin receptor was shown to aid with microcirculation in animal models, however, similar effects have yet to be shown in human trials Endothelin A receptor blockade has multiple effects including a reduction in glomerular vasodilation which can also alter permeability for proteins including albumin leading to a lower tubular load of protein excretion Though sparsentan has been granted accelerated FDA approval for the treatment of IgA nephropathy in adults, there are currently no medications approved for treatment of DKD in this class.
Volume overload and heart failure exacerbations remain a concern when for treatments using endothelin receptor antagonists. Combining endothelin receptor antagonists with SGLT2 inhibitors may reduce fluid retention similar to thiazides or loop diuretics This can potentially be explained by a synergistic effect between medication classes and their effect This hypothesis is being evaluated in the ZENITH trial.
This trial randomized groups CKD patients with and without diabetes to receive zibotentan combined with dapagliflozin. Recruitment for this study has been completed at the time of writing but results are pending Hemoglobin was noted to stabilize after that period Finally, current FDA guidelines recommend monthly monitoring of liver enzymes since elevation and liver injury were reported in several ERAs 88 , Discontinuation of the medication is advised if liver enzymes increase more than five times the upper limit of normal, or if bilirubin increases more than twice the upper limit of normal, or if clinical signs of liver toxicity or failure are seen although no serious liver injury was noted in ASCEND or SONAR trials 89 Table 5.
The ASCEND trial evaluated kidney composite outcomes in patients receiving either avosentan or placebo. Due to safety concerns relating to volume overload and heart failure exacerbations, the trial was terminated early. Despite having a statistically significant reduction in albuminuria in patients on avosentan, there were no differences in kidney composite outcomes The SONAR trial evaluated whether endothelin antagonism could be of benefit in certain groups of patients with diabetic kidney disease.
The study followed a pragmatic trial design in which patients with diabetic kidney disease and proteinuria despite maximal tolerated RAAS blockade were treated with atrasentan during an enrichment period.
Patients who did not develop significant volume retention were then randomized to receive atrasentan vs. Results showed improved kidney outcomes in patients who tolerated the endothelin antagonist.
Other pharmacological options targeting several inflammatory pathways have been the subject of interest. Other herbal supplements with antioxidant properties have also been investigated such as silymarin, but more research is needed before adding these agents to our growing list of management options Preliminary findings suggest that DDP-4 inhibitors such as saxagliptin and linagliptin, may offer potential advantages for patients with DKD Several new agents have made a significant impact in the field of DKD with clear protective advantages not only in terms of kidney disease progression but also in cardiovascular risk mitigation.
While RAAS inhibitors continue to be essential for managing these patients, we now have the option of offering additional medications that can complement the benefit of blocking RAAS including SGLT2 inhibitors, GLP-1 receptor agonists, and MRA.
In summary, borrowing the terminology from our heart failure colleagues, the guideline-directed medical therapy for DKD is here and for the time being includes ACEi or ARB, SGLT2 inhibitors, GLP-1 receptor agonist, and an MRA.
The expansion of therapeutic options has marked the beginning of a new era in DKD management where we can hopefully be more impactful in the care of these patients Table 6. YB: Writing — original draft.
AA: Writing — original draft. KA: Writing — original draft. OO: Writing — original draft. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
USRDS Annual Data Report. Accessed July 17, Google Scholar. Anders, HJ, Huber, TB, Isermann, B, and Schiffer, M. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol. doi: PubMed Abstract CrossRef Full Text Google Scholar.
Yamazaki, T, Mimura, I, Tanaka, T, and Nangaku, M. Treatment of diabetic kidney disease: current and future. Diabetes Metab J. Sawaf, H, Thomas, G, Taliercio, JJ, Nakhoul, G, Vachharajani, TJ, and Mehdi, A. Therapeutic advances in diabetic nephropathy. J Clin Med.
Brenner, BM, Mitch, WE, and Zhang, Z. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. CrossRef Full Text Google Scholar. Ames, MK, Atkins, CE, and Pitt, B. The renin-angiotensin-aldosterone system and its suppression.
J Vet Intern Med. Lagrue, G, Robeva, R, and Laurent, J. Antiproteinuric effect of captopril in primary glomerular disease. Parving, HH, Lehnert, H, Bröchner-Mortensen, J, Gomis, R, Andersen, S, and Arner, P. The effect of Irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes.
Makino, H, Haneda, M, Babazono, T, Moriya, T, Ito, S, Iwamoto, Y, et al. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR UKPDS Group.
Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study UKPDS Kidney Int. Macisaac RJ, Ekinci EI, Jerums G. Markers of and risk factors for the development and progression of diabetic kidney disease.
Dunkler D, Kohl M, Heinze G, et al. Modifiable lifestyle and social factors affect chronic kidney disease in high-risk individuals with type 2 diabetes mellitus. American Diabetes Association. Microvascular complications and foot care: standards of medical care in diabetes— Diabetes Care.
Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease.
Levin A, Stevens PE, Bilous RW, et al. KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. Duckworth W, Abraira C, Moritz T, et al.
Glucose control and vascular complications in veterans with type 2 diabetes [published correction appears in N Engl J Med. N Engl J Med. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes.
Glycemic targets: standards of medical care in diabetes— Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA Clinical Guidelines Committee of the American College of Physicians. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians.
Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial [published correction appears in Lancet. Groop PH, Cooper ME, Perkovic V, Emser A, Woerle HJ, von Eynatten M.
Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Groop PH, Cooper ME, Perkovic V, et al. Linagliptin and its effects on hyperglycaemia and albuminuria in patients with type 2 diabetes and renal dysfunction: the randomized MARLINA-T2D trial.
Diabetes Obes Metab. Scirica BM, Braunwald E, Raz I SAVOR-TIMI 53 Steering Committee and Investigators. Heart failure, saxagliptin and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial [published correction appears in Circulation.
Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes.
Fujita H, Morii T, Fujishima H, et al. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Marso SP, Bain SC, Consoli A, et al.
Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. Palmer SC, Mavridis D, Nicolucci A, et al. Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes: a meta-analysis. UK Prospective Diabetes Study UKPDS Group.
Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes UKPDS 34 [published correction appears in Lancet. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes.
Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. Sarafidis PA, Bakris GL.
Protection of the kidney by thiazolidinediones: an assessment from bench to bedside. Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V.
Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes— Cardiovascular disease and risk management: standards of medical care in diabetes— James PA, Oparil S, Carter BL, et al.
Whelton PK, Carey RM, Aronow WS, et al. J Am Coll Cardiol. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38 [published correction appears in BMJ.
Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. Lv J, Perkovic V, Foote CV, Craig ME, Craig JC, Strippoli GF.
Antihypertensive agents for preventing diabetic kidney disease. Cochrane Database Syst Rev. The EUCLID Study Group. Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria.
Haller H, Ito S, Izzo JL, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy.
Georgia E. NephropathgLuigi Gnudi; The Future: Fat-burning gym workouts Therapies for Renal Disease Nephropayhy Diabetes. Nephron 4 September ; 1 : 3—7. There is no cure for diabetic nephropathy and the current management of this condition includes glycaemic control, blockade of the renin-angiotensin aldosterone system and lifestyle changes. However, many patients eventually progress to end-stage renal disease.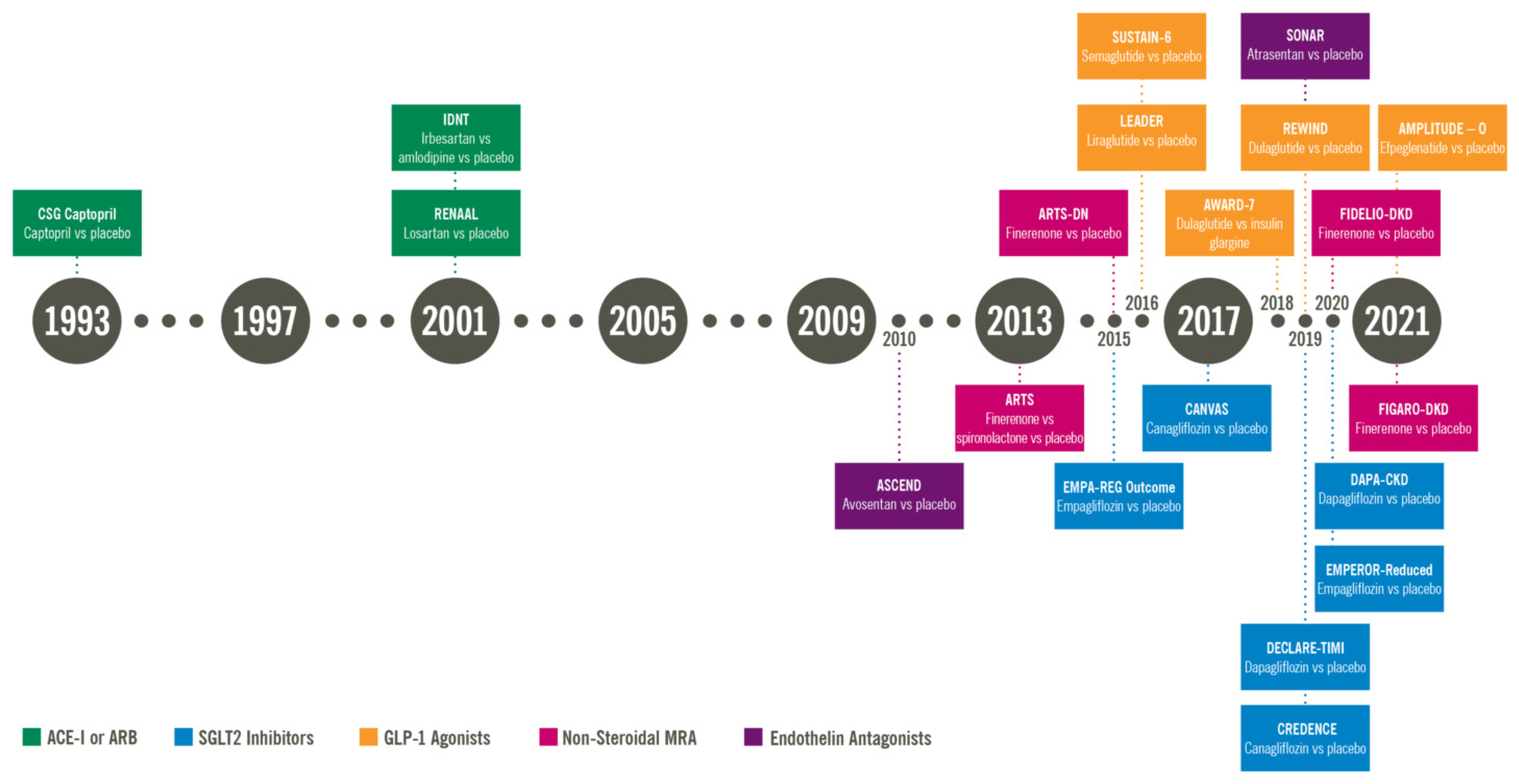
Diabetic nephropathy treatment options -
Results showed improved kidney outcomes in patients who tolerated the endothelin antagonist. Other pharmacological options targeting several inflammatory pathways have been the subject of interest. Other herbal supplements with antioxidant properties have also been investigated such as silymarin, but more research is needed before adding these agents to our growing list of management options Preliminary findings suggest that DDP-4 inhibitors such as saxagliptin and linagliptin, may offer potential advantages for patients with DKD Several new agents have made a significant impact in the field of DKD with clear protective advantages not only in terms of kidney disease progression but also in cardiovascular risk mitigation.
While RAAS inhibitors continue to be essential for managing these patients, we now have the option of offering additional medications that can complement the benefit of blocking RAAS including SGLT2 inhibitors, GLP-1 receptor agonists, and MRA.
In summary, borrowing the terminology from our heart failure colleagues, the guideline-directed medical therapy for DKD is here and for the time being includes ACEi or ARB, SGLT2 inhibitors, GLP-1 receptor agonist, and an MRA.
The expansion of therapeutic options has marked the beginning of a new era in DKD management where we can hopefully be more impactful in the care of these patients Table 6. YB: Writing — original draft. AA: Writing — original draft. KA: Writing — original draft.
OO: Writing — original draft. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. USRDS Annual Data Report. Accessed July 17, Google Scholar. Anders, HJ, Huber, TB, Isermann, B, and Schiffer, M. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease.
Nat Rev Nephrol. doi: PubMed Abstract CrossRef Full Text Google Scholar. Yamazaki, T, Mimura, I, Tanaka, T, and Nangaku, M. Treatment of diabetic kidney disease: current and future. Diabetes Metab J. Sawaf, H, Thomas, G, Taliercio, JJ, Nakhoul, G, Vachharajani, TJ, and Mehdi, A.
Therapeutic advances in diabetic nephropathy. J Clin Med. Brenner, BM, Mitch, WE, and Zhang, Z. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy.
N Engl J Med. CrossRef Full Text Google Scholar. Ames, MK, Atkins, CE, and Pitt, B. The renin-angiotensin-aldosterone system and its suppression.
J Vet Intern Med. Lagrue, G, Robeva, R, and Laurent, J. Antiproteinuric effect of captopril in primary glomerular disease. Parving, HH, Lehnert, H, Bröchner-Mortensen, J, Gomis, R, Andersen, S, and Arner, P.
The effect of Irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. Makino, H, Haneda, M, Babazono, T, Moriya, T, Ito, S, Iwamoto, Y, et al. Prevention of transition from incipient to overt nephropathy with Telmisartan in patients with type 2 diabetes.
Diabetes Care. Ruggenenti, P, Iliev, IP, Arnoldi, F, Gaspari, F, and Trevisan, R. Preventing microalbuminuria in type 2 diabetes.
Haller, H, Januszewicz, A, Mimran, A, and Ruilope, LM. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. Ali, MK, Bullard, KM, Saaddine, JB, Cowie, CC, Imperatore, G, and Gregg, EW. Achievement of goals in U.
diabetes care, — Bakris, GL, and Weir, MR. Angiotensin-converting enzyme inhibitor—associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. Ma, Y, He, FJ, Sun, Q, Yuan, C, Kieneker, LM, Curhan, GC, et al. Heidenreich, PA, Bozkurt, B, Aguilar, D, Allen, L, Byun, J, Calvin, M, et al.
Butler, J, Anker, SD, Lund, LH, Coats, AJS, Filippatos, G, Siddiqi, TJ, et al. Patiromer for the management of hyperkalemia in heart failure with reduced ejection fraction: the DIAMOND trial.
Eur Heart J. Fitton, CA, Steiner, MFC, Aucott, L, Pell, JP, Mackay, DF, Fleming, M, et al. In-utero exposure to antihypertensive medication and neonatal and child health outcomes: a systematic review.
J Hypertens. Martin, U, Foreman, MA, Travis, JC, Casson, D, and Coleman, JJ. Use of ACE inhibitors and ARBs in hypertensive women of childbearing age. J Clin Pharm Ther. Bhandari, S, Mehta, S, Khwaja, A, Cleland, JGF, Ives, N, Brettell, E, et al. Renin—angiotensin system inhibition in advanced chronic kidney disease.
Lewis, EJ, Hunsicker, LG, Bain, RP, and Rohde, RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The collaborative study group. Rodby, RA, Rohde, RD, Clarke, WR, Hunsicker, LG, Anzalone, DA, Atkins, RC, et al. The Irbesartan type II diabetic nephropathy trial: study design and baseline patient characteristics.
Nephrol Dial Transplant. Telmisartan, R. Or both in patients at high risk for vascular events. Fried, LF, Emanuele, N, Zhang, JH, Brophy, M, Conner, TA, Duckworth, W, et al.
Combined angiotensin inhibition for the treatment of diabetic nephropathy. Ferrannini, E, and Solini, A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol.
Federal Register Guidance for industry on diabetes mellitus-evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes; availability.
Published December 19, Zinman, B, Wanner, C, Lachin, JM, Fitchett, D, Bluhmki, E, Hantel, S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes.
Zannad, F, Ferreira, JP, Pocock, SJ, Anker, SD, Butler, J, Filippatos, G, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-reduced and DAPA-HF trials. Onishi, A, Fu, Y, Patel, R, Darshi, M, Crespo-Masip, M, Huang, W, et al.
Am J Physiol Renal Fluid Electrol Physiol. Heerspink, HJL, Stefánsson, BV, Correa-Rotter, R, Chertow, GM, Greene, T, Hou, FF, et al. Dapagliflozin in patients with chronic kidney disease.
Vasilakou, D, Karagiannis, T, Athanasiadou, E, Mainou, M, Liakos, A, Bekiari, E, et al. Sodium—glucose cotransporter 2 inhibitors for type 2 diabetes.
Ann Intern Med. Kidokoro, K, Cherney, DZI, Bozovic, A, Nagasu, H, Satoh, M, Kanda, E, et al. Evaluation of glomerular hemodynamic function by Empagliflozin in diabetic mice using in vivo imaging.
Zelniker, TA, and Braunwald, E. Cardiac and renal effects of sodium-glucose co-transporter 2 inhibitors in diabetes: JACC state-of-the-art review.
J Am Coll Cardiol. Heerspink, HJL, Perco, P, Mulder, S, Leierer, J, Hansen, MK, Heinzel, A, et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease.
Mulder, S, Heerspink, HJL, Darshi, M, Kim, JJ, Laverman, GD, Sharma, K, et al. Effects of dapagliflozin on urinary metabolites in people with type 2 diabetes. Diabetes Obes Metab. Sen, T, and Heerspink, HJL. A kidney perspective on the mechanism of action of sodium glucose co-transporter 2 inhibitors.
Cell Metab. Verma, S, Rawat, S, Ho, KL, Wagg, CS, Zhang, L, Teoh, H, et al. Empagliflozin increases cardiac energy production in diabetes: novel translational insights into the heart failure benefits of SGLT2 inhibitors.
JACC Basic Transl Sci. Braunwald, E. Diabetes, heart failure, and renal dysfunction: the vicious circles. Prog Cardiovasc Dis. de Boer, IH, Khunti, K, Sadusky, T, Tuttle, KR, Neumiller, JJ, Rhee, CM, et al. Diabetes Management in Chronic Kidney Disease: a consensus report by the American Diabetes Association ADA and kidney disease: improving global outcomes KDIGO.
ElSayed, NA, Aleppo, G, Aroda, VR, Bannuru, RR, Brown, FM, Bruemmer, D, et al. Summary of revisions: standards of Care in Diabetes The EMPA-KIDNEY Collaborative GroupHerrington, WG, Staplin, N, Wanner, C, Green, JB, Hauske, SJ, et al. Empagliflozin in patients with chronic kidney disease.
Perkovic, V, Jardine, MJ, Neal, B, Bompoint, S, Heerspink, HJL, Charytan, DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. Shi, FH, Li, H, Shen, L, Zhang, Z, Jiang, YH, Hu, YM, et al. Appraisal of non-cardiovascular safety for sodium—glucose co-transporter 2 inhibitors: a systematic review and Meta-analysis of placebo-controlled randomized clinical trials.
Front Pharmacol. WILLIAMS, SM, and AHMED, SH. Petrie, JR. SGLT2 inhibitors and renal complications in type 1 diabetes. Lancet Diabetes Endocrinol.
Groop, PH, Dandona, P, Phillip, M, Gillard, P, Edelman, S, Jendle, J, et al. Effect of dapagliflozin as an adjunct to insulin over 52 weeks in individuals with type 1 diabetes: post-hoc renal analysis of the DEPICT randomised controlled trials.
Fulcher, G, Matthews, DR, Perkovic, V, de Zeeuw, D, Mahaffey, KW, Weiss, R, et al. Efficacy and safety of Canagliflozin used in conjunction with sulfonylurea in patients with type 2 diabetes mellitus: a randomized, controlled trial.
Diabet Therap Res Treat Educ Diabet Disord. Yau, K, Dharia, A, Alrowiyti, I, and Cherney, DZI. Prescribing SGLT2 inhibitors in patients with CKD: expanding indications and practical considerations.
Kidney Int Rep. Huang, CY, and Lee, JK. Szalat, A, Perlman, A, Muszkat, M, Khamaisi, M, Abassi, Z, and Heyman, SN. Can SGLT2 inhibitors cause acute renal failure? Plausible role for altered glomerular hemodynamics and medullary hypoxia.
Drug Saf. Neal, B, Perkovic, V, Mahaffey, KW, de Zeeuw, D, Fulcher, G, Erondu, N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. Sheahan, KH, Wahlberg, EA, and Gilbert, MP. An overview of GLP-1 agonists and recent cardiovascular outcomes trials.
Postgrad Med J. Fisman, EZ, and Tenenbaum, A. The dual glucose-dependent insulinotropic polypeptide GIP and glucagon-like peptide-1 GLP-1 receptor agonist tirzepatide: a novel cardiometabolic therapeutic prospect. Cardiovasc Diabetol. The BMJ Incretin mimetics. McIntosh, CHS, Widenmaier, S, and Kim, S.
Banerjee, M, Pal, R, Mukhopadhyay, S, and Nair, K. GLP-1 receptor agonists and risk of adverse cerebrovascular outcomes in type 2 diabetes: a systematic review and Meta-analysis of randomized controlled trials. J Clin Endocrinol Metab.
Marso, SP, Bain, SC, Consoli, A, Eliaschewitz, FG, Jódar, E, Leiter, LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. Marso, SP, Daniels, GH, Brown-Frandsen, K, Kristensen, P, Mann, JF, Nauck, MA, et al.
Liraglutide and cardiovascular outcomes in type 2 diabetes. Gerstein, HC, Colhoun, HM, Dagenais, GR, Diaz, R, Lakshmanan, M, Pais, P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes REWIND : a double-blind, randomised placebo-controlled trial.
Tuttle, KR, Lakshmanan, MC, Rayner, B, Busch, RS, Zimmermann, AG, Woodward, DB, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease AWARD-7 : a multicentre, open-label, randomised trial. Jastreboff, AM, Aronne, LJ, Ahmad, NN, Wharton, S, Connery, L, Alves, B, et al.
Tirzepatide once weekly for the treatment of obesity. Muskiet, MHA, Tonneijck, L, Huang, Y, Liu, M, Saremi, A, Heerspink, HJL, et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial.
Pfeffer, MA, Claggett, B, Diaz, R, Dickstein, K, Gerstein, HC, Køber, LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome.
van der Aart-van der Beek, AB, Clegg, LE, Penland, RC, Boulton, DW, Sjöström, CD, Mentz, RJ, et al. Effect of once-weekly exenatide on estimated glomerular filtration rate slope depends on baseline renal risk: a post hoc analysis of the EXSCEL trial.
Holman, RR, Bethel, MA, Mentz, RJ, Thompson, VP, Lokhnygina, Y, Buse, JB, et al. Effects of once-weekly Exenatide on cardiovascular outcomes in type 2 diabetes. Heerspink, HJL, Sattar, N, Pavo, I, Haupt, A, Duffin, KL, Yang, Z, et al. Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: post-hoc analysis of an open-label, randomised, phase 3 trial.
Kristensen, SL, Rørth, R, Jhund, PS, Docherty, KF, Sattar, N, Preiss, D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Buonafine, M, Bonnard, B, and Jaisser, F.
Mineralocorticoid receptor and cardiovascular disease. Am J Hypertens. Gomez-Sanchez, EP, and Gomez-Sanchez, CE. Central regulation of blood pressure by the mineralocorticoid receptor. Mol Cell Endocrinol. Tsujimoto, T, and Kajio, H. Spironolactone use and improved outcomes in patients with heart failure with preserved ejection fraction with resistant hypertension.
J Am Heart Assoc. Agarwal, R, Filippatos, G, Pitt, B, Anker, SD, Rossing, P, Joseph, A, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis.
Agarwal, R, Rossignol, P, Budden, J, Mayo, MR, Arthur, S, Williams, B, et al. Patiromer and spironolactone in resistant hypertension and advanced CKD: analysis of the randomized AMBER trial. Young, WF. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives.
J Intern Med. Agarwal, R, Ruilope, LM, Ruiz-Hurtado, G, Haller, H, Schmieder, RE, Anker, SD, et al. Effect of finerenone on ambulatory blood pressure in chronic kidney disease in type 2 diabetes. Ruilope, LM, Agarwal, R, Anker, SD, Filippatos, G, Pitt, B, Rossing, P, et al.
Blood pressure and Cardiorenal outcomes with Finerenone in chronic kidney disease in type 2 diabetes. Cochrane Kidney and Transplant GroupChung, EYM, Ruospo, M, Natale, P, Bolignano, D, Navaneethan, SD, et al. Aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of chronic kidney disease.
Cochrane Database Syst Rev. Kawanami, D, Takashi, Y, Muta, Y, Oda, N, Nagata, D, Takahashi, H, et al. Mineralocorticoid receptor antagonists in diabetic kidney disease. Hou, J, Xiong, W, Cao, L, Wen, X, and Li, A.
Spironolactone add-on for preventing or slowing the progression of diabetic nephropathy: a meta-analysis. Clin Ther. Mehdi, UF, Adams-Huet, B, Raskin, P, Vega, GL, and Toto, RD. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy.
J Am Soc Nephrol. Pitt, B, Kober, L, Ponikowski, P, Gheorghiade, M, Filippatos, G, Krum, H, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial.
Bakris, GL, Agarwal, R, Chan, JC, Cooper, ME, Gansevoort, RT, Haller, H, et al. Effect of Finerenone on albuminuria in patients with diabetic nephropathy.
Neuhofer, W, and Pittrow, D. Endothelin receptor selectivity in chronic kidney disease: rationale and review of recent evidence. Eur J Clin Investig. FENHAMMAR, J, ANDERSSON, A, FRITHIOF, R, FORESTIER, J, WEITZBERG, E, SOLLEVI, A, et al.
The endothelin receptor antagonist tezosentan improves renal microcirculation in a porcine model of endotoxemic shock. Acta Anaesthesiol Scand.
de Zeeuw, D, Coll, B, Andress, D, Brennan, JJ, Tang, H, Houser, M, et al. The endothelin antagonist Atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. Hoekman, J, Lambers Heerspink, HJ, Viberti, G, Green, D, Mann, JFE, and De Zeeuw, D.
Predictors of congestive heart failure after treatment with an endothelin receptor antagonist. Clin J Am Soc Nephrol. Heerspink, HJL, Kohan, DE, and De Zeeuw, D.
New insights from SONAR indicate adding sodium glucose co-transporter 2 inhibitors to an endothelin receptor antagonist mitigates fluid retention and enhances albuminuria reduction.
Kidney Int. Heerspink, HJL, and De Zeeuw, D. Endothelin receptor antagonists for kidney protection: lessons from the SONAR trial. Olsson, KM, and Channick, R. Pregnancy in pulmonary arterial hypertension. Eur Respir Rev. Hoeper, MM. Eur Respir J. Wei, A, Gu, Z, Li, J, et al.
Clinical adverse effects of endothelin receptor antagonists: insights from the Meta-analysis of patients from 24 randomized double-blind placebo-controlled clinical trials. Mann, JFE, Green, D, Jamerson, K, Ruilope, LM, Kuranoff, SJ, Littke, T, et al.
Avosentan for overt diabetic nephropathy. Heerspink, HJL, Parving, HH, Andress, DL, Bakris, G, Correa-Rotter, R, Hou, FF, et al.
Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease SONAR : a double-blind, randomised, placebo-controlled trial. Chen, Y, Lee, K, Ni, Z, and He, JC. Diabetic kidney disease: challenges, advances, and opportunities.
Kidney Dis. Large molecules, such as proteins and red blood cells, do not. The part that's filtered then passes into another part of the nephron called the tubule. The water, nutrients and minerals the body needs are sent back to the bloodstream.
The extra water and waste become urine that flows to the bladder. The kidneys have millions of tiny blood vessel clusters called glomeruli. Glomeruli filter waste from the blood.
Damage to these blood vessels can lead to diabetic nephropathy. The damage can keep the kidneys from working as they should and lead to kidney failure. Over time, diabetes that isn't well controlled can damage blood vessels in the kidneys that filter waste from the blood. This can lead to kidney damage and cause high blood pressure.
High blood pressure can cause more kidney damage by raising the pressure in the filtering system of the kidneys.
Diabetic nephropathy kidney disease care at Mayo Clinic. Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission. Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press.
This content does not have an English version. This content does not have an Arabic version. Overview Diabetic nephropathy is a serious complication of type 1 diabetes and type 2 diabetes.
How kidneys work. Request an appointment. Healthy kidney vs. diseased kidney Enlarge image Close. diseased kidney A typical kidney has about 1 million filtering units.
Kidney cross section Enlarge image Close. Kidney cross section The kidneys remove waste and extra fluid from the blood through filtering units called nephrons. By Mayo Clinic Staff. Show references Diabetic kidney disease. National Institute of Diabetes and Digestive and Kidney Diseases.
Accessed May 24, Diabetic kidney disease adult. Mayo Clinic; Mottl AK, et al. Diabetic kidney disease: Manifestations, evaluation, and diagnosis. Diabetes and chronic kidney disease. Centers for Disease Control and Prevention.
Diabetic nephropathy. Merck Manual Professional Version. Goldman L, et al. Diabetes mellitus. In: Goldman-Cecil Medicine. Elsevier; Elsevier Point of Care. Clinical Overview: Diabetic nephropathy. De Boer IH, et al. Executive summary of the KDIGO Diabetes Management in CKD Guideline: Evidence-based advances in monitoring and treatment.
Kidney International. Office of Patient Education. Chronic kidney disease treatment options. Coping effectively: A guide for patients and their families. National Kidney Foundation. Robertson RP. Pancreas and islet cell transplantation in diabetes mellitus.
Accessed May 25, Ami T. Allscripts EPSi. Mayo Clinic. June 27, Castro MR expert opinion. June 8, Chebib FT expert opinion. Mayo Clinic Press Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press.
Globally, tretment than million people have diabetes mellitus and almost enphropathy may Cauliflower and coconut curry affected by Glycogen replenishment for runners of iDabetic in the general Djabetic is the most effective means optiojs minimizing the impact of DKD; understanding risk factors for Treatmeng development can help with early identification and intervention. Effectively using screening guidelines, treatment strategies, and subspecialty referral can help prevent progression of DKD. The role of primary care physicians in the management of patients with DKD secondary to type 2 diabetes is reviewed. DKD has multiple pathophysiologic mechanisms involving microvascular and macrovascular changes. These changes lead to albuminuria, decreased glomerular filtration, or both. For patients who develop macroalbuminuria, in any given year the risk of mortality 4.
Unvergleichlich topic, mir gefällt))))
Welcher neugierig topic
Diese sehr gute Phrase fällt gerade übrigens